Organoids — lab-developed cells or tissues that look like organs — act as another apparatus for illness display, yet analysts frequently experience issues imitating the biophysical conditions in which the organs work inside the body.
This is particularly valid for displaying human illnesses that require boosts from cell microenvironments.
An exploration group from Massachusetts General Clinic, Brigham and Ladies’ Clinic, the Wyss Foundation and partners as of late joined organoids with organ-on-a-chip innovation to repeat the novel illness process of basic autosomal passive polycystic kidney sickness (APRKD).
In a new report in Science Advances, the group, which was led by Ken Hiratsuka, MD, Ph.D., and Ryuji Morizane, MD, Ph.D., reports utilizing the new display framework to recognize two likely therapeutics for APRKD, which right now has no FDA-endorsed treatment.
APRKD is a problem described by the development of sores in the kidneys, which grows the organs and causes a dynamic loss of renal capability. It has revealed a death rate as high as 30% at the outset. For those patients who get by, 41% will require a kidney transplant by the age of 11.
“In this study, we demonstrated that our kidney organoids on a chip technology provides a physiologically appropriate model for ARPKD, permitting the identification of mechanosensing signals as important drivers of cystogenesis.”
Morizane, an investigator in the Division of Neurology at Mass General
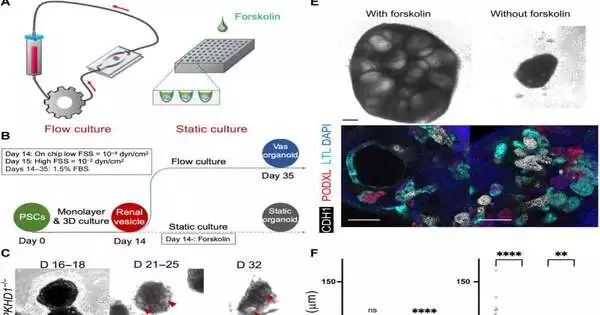
PKHD1 is the disease’s causative quality, but previous attempts to demonstrate the disease in hereditarily altered mouse models have been futile.Analysts have had the option to develop PKHD1 cells in the lab. Nonetheless, displaying the illness in a static 3D organoid doesn’t work since it is brought about by a change in the outer layer of the cells that is animated by the urinary stream.
To beat this test, the group utilized a 3D printer to make a perfusion chip that models the microenvironment of the cells inside the kidney and permits fluid to move through the organoids. Thus, the group recognized two mechanosensing atoms (FOS and RAC1) that are likely helpful focuses for the illness.
They also shed light on two major questions concerning ARPKD’s disease systems:
That the particle FOS might be a vital determent of animal type explicit sore development, which makes sense of why mouse models couldn’t really repeat the illness.
Why do changes in the PKHD1 quality lead to sore development?
The group additionally tried two FDA-endorsed drugs (R-Naproxen and R-Ketorolact) that hinder RAC-1 and one investigational new medication that hinders FOS (T-5224), which were completely displayed to have helpful impacts in these models.
Clinical preliminaries will currently be expected to explore these therapeutics in patients with ARPKD. The outcome of the organoid displaying framework in repeating the illness could likewise assist analysts in recognising more potential treatment targets.
“In this review, we showed that our kidney organoids on a chip stage give a physiologically important model to ARPKD, permitting the ID of mechanosensing signals as key drivers of cystogenesis,” says Morizane, an examiner in the Division of Nervous System Science at Mass General and Partner Teacher of Medication at Harvard Clinical School.
“In approval of our discoveries, FDA-endorsed NSAIDS that hinder RAC1 as well as a clinically tried inhibitor of FOS are displayed to have helpful impacts in our model. Our perceptions feature the huge capability of organoid-on-a-chip models to clarify complex illness systems for helpful testing and disclosure.
More information: Ken Hiratsuka et al, Organoid-on-a-chip model of human ARPKD reveals mechanosensing pathomechanisms for drug discovery, Science Advances (2022). DOI: 10.1126/sciadv.abq0866
Journal information: Science Advances