Nanoparticles are used in preclinical research to attach invulnerable enacting particles to cancers, sharpening them for immunotherapy.
Researchers have fostered a nanotechnology stage that can impact the manner in which the resistant framework sees strong growth cells, making them more responsive to immunotherapy. This versatile, invulnerable transformation approach has the potential for expansive application across numerous disease types, as indicated by preclinical discoveries.
The review discusses how this stage was used to incorrectly attach an enactment particle to the outer layer of cancer cells, causing a resistant reaction in both in vivo and in vitro models.It will be distributed today (November 10) in the journal Nature Nanotechnology. Wen Jiang, M.D., Ph.D., right-hand teacher of radiation oncology, and Betty Kim, M.D., Ph.D., teacher of neurosurgery, co-led the review, which was led by a group of scientists at The College of Texas MD Anderson Malignant Growth Place.
“It might be possible to reach closer to the maximum level of activity from immunotherapy medications with tumors that have not previously responded well if we are able to transfer and validate this technique in the clinic.”
Wen Jiang, M.D., Ph.D., assistant professor of Radiation Oncology,
“With this new stage, we presently have a system to change a strong cancer, in some measure immunologically, to look like a hematological growth, which frequently has a much higher reaction rate to immunotherapy therapies,” Jiang said. “Assuming we can decipher and approve this methodology in the center, it might empower us to draw nearer to the greatest degree of movement from immunotherapy drugs with malignant growths that poor people have traditionally answered well.”
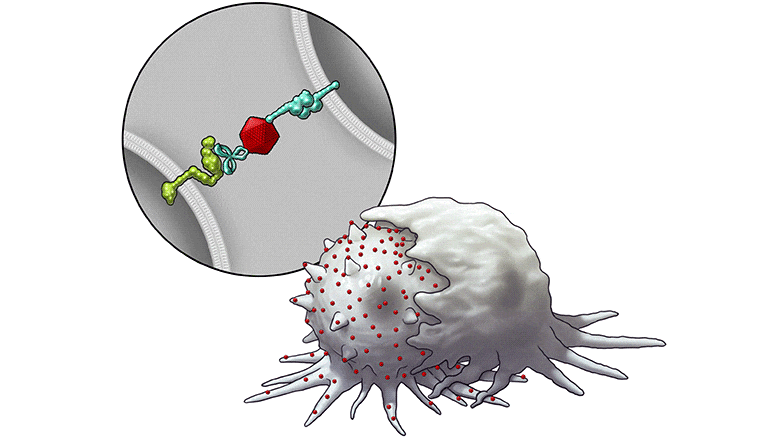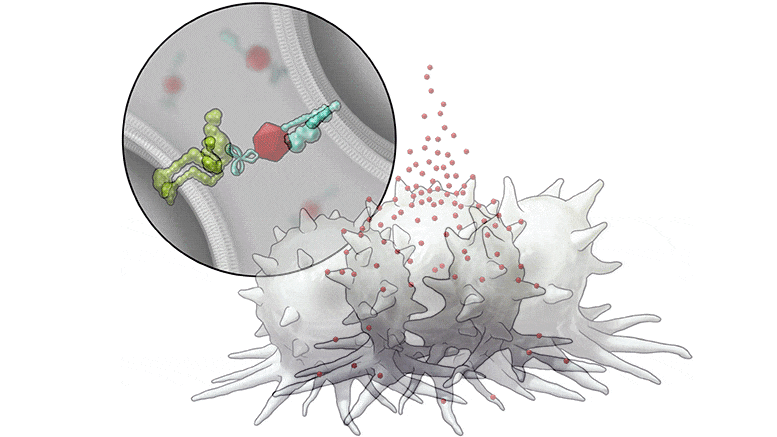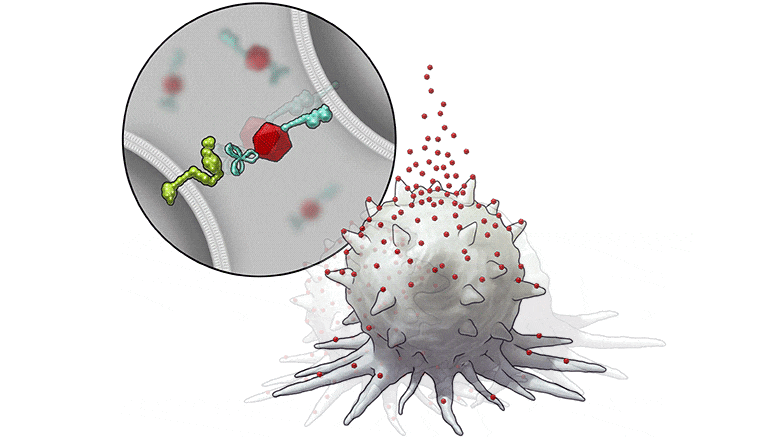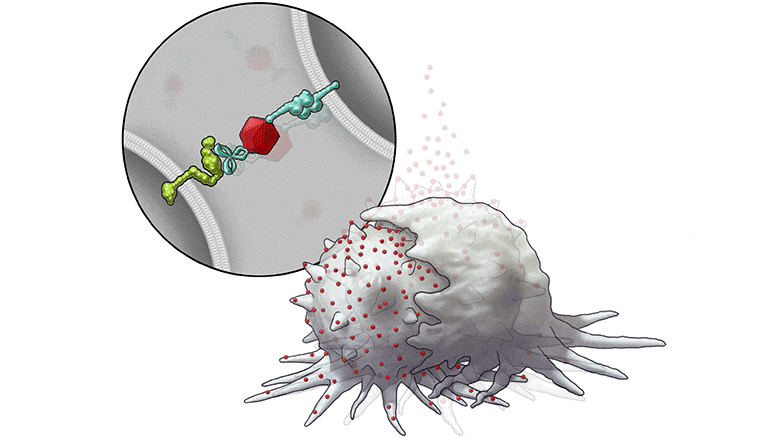
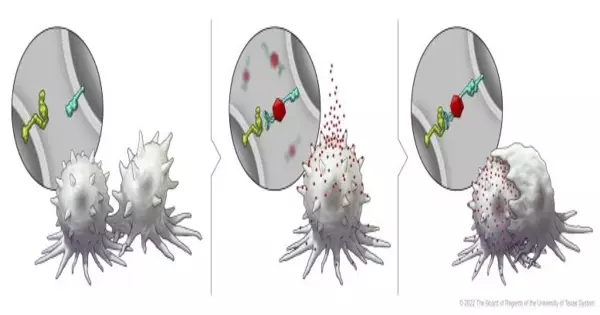
In this series of illustrations, the immune cell does not initially recognize the cancer cell. After BiTN particles (red), which include the “eat me” signal (teal), are attached to the cancer cell, the immune cell recognizes the cell to ingest it.
Immunotherapy has a high response rate in blood-borne malignant growths such as leukemia and lymphoma; however, success has varied across strong cancers.Researchers have been attempting to further comprehend the instruments restricting a superior reaction. One clarification is that the differing articulation of resistant administrative atoms in blood disease versus strong growth cells influences how they interface with invulnerable cells.
The flagging lymphocytic enactment particle relative 7 (SLAMF7) receptor is basic in actuating the body’s safe cells against disease cells, acting as an “eat me” signal. Be that as it may, it is found solely on the outer layer of blood malignant growth cells and not in strong cancer cells, making it an alluring objective for the scientists’ safe change approach.
To advance the declaration of SLAMF7 on strong growth cells, the scientists fostered their bispecific cancer-changing nanoconjugate (BiTN) stage. These nanosystems are designed with one particle to tie to the outer layer of designated growth cells and a second atom to initiate a resistant reaction.
In this review, the scientists utilized BiTN with SLAMF7 and a HER2-perceiving neutralizer to target HER2-positive bosom disease cells. In research center models, the nanoconjugate effectively connected SLAMF7 to the bosom disease cells, bringing about phagocytosis, or ingestion, by resistant cells. The methodology also sharpened the bosom malignant growth cells for treatment with an antagonist of CD47 neutralizer, which obstructs cancer cells’ “don’t eat me” signal to induce additional increment reactions in strong cancers.
As per the creators, quite possibly one of the most intriguing things about this stage is its wide expected applications. The methodology wouldn’t be well defined for one malignant growth type or one administrative particle, but rather it could possibly be a general procedure for a few different strong cancer types. As a proof of concept, the creators likewise created BiTN with folate rather than the counter-HER2-immune response to target triple-negative breast malignant growth with comparative outcomes.
“Since these are designed developments, this can be utilized as a plug-and-play way to deal with consolidating different growths focusing on specialists or resistant particles onto the outer layer of the nanoparticle,” Kim said. “For patients with strong cancers that poorly responded to immunotherapy, we consider this to be an additional benefit to focus on the piece of the growth that didn’t respond.”
Reference: “Immunological conversion of solid tumours using a bispecific nanobioconjugate for cancer immunotherapy” 10 November 2022, Nature Nanotechnology.
DOI: 10.1038/s41565-022-01245-7
The study was supported in part by the Susan G. Komen Foundation Career Catalyst Research Grant, the National Cancer Institute/National Institutes of Health (1K08 CA241070, P30 CA016672), and the United States Department of Defense. A full list of co-authors and disclosures can be found in the full paper.