An exploration group, a subsidiary of UNIST, has divulged an original strategy to create a specific anticancer forerunner substance that targets and kills malignant growth cells. This pivotal technique, already existing just in principle, has now been tentatively demonstrated, interestingly opening up additional opportunities in the improvement of imaginative medications through a broad examination of the impacts of anticancer antecedents on the human body.
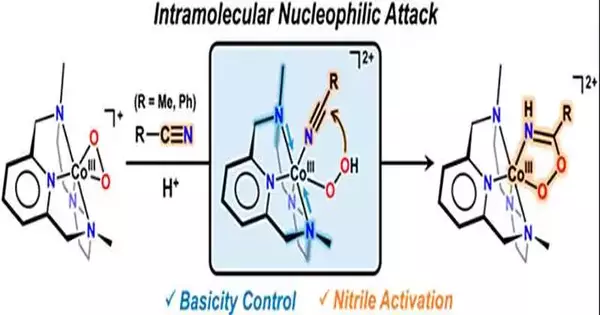
Driven by Teacher Jaeheung Cho of the Division of Science at UNIST, the exploration group has effectively exhibited that the combination of hydroxymato cobalt (III), a potential competitor substance for anticancer forerunners, includes the response of metal-dynamic oxygen species with nitrile. Not at all like past examinations that depended on costly, weighty metals, this new technique uses practical metals and works at lower temperatures.
The work is distributed in the journal JACS Au.
“This study reveals the underlying mechanisms of metal-active oxygen species in nitrile activation, laying the groundwork for the future development of nitrile activating catalysts.”
Professor Jaeheung Cho of the Department of Chemistry at UNIST,
Nitrile, a compound broadly utilized in drugs and horticultural pesticides, has demonstrated testing to blend. Nonetheless, the examination group has now affirmed that the response among nitriles and cobalt-hydroperoxo species, a kind of metal-dynamic oxygen species, prompts the blend of peroxyimidate to cobalt (III). This finding uncovers that peroxyimidateto cobalt (III) is a middle substance shaped during the compound response, eventually delivering hydroxymiteto cobalt (III).
To orchestrate cobalt (III)-peroxyimidato edifices, the examination group presented another species known as acobalt (III)-hydroperoxo particulars. Surprisingly, they found that the response happens when hydroperoxo is nucleophilic, followed by nitrile. Besides, it was seen that the expansion of a base to peroxymidato cobalt (III) changes it into hydroxymito cobalt (III), empowering the blend of forerunners.
The exploration group put specific accentuation on the meaning of the basicity of metal-dioxygen determinations, specifically the metal-(hydro)peroxo [M-O2(H)] complex species. By controlling the molecules bound to the cobalt-hydroperoxo species that didn’t respond with nitrile, they effectively expanded basicity, empowering quick responses even at low temperatures.
To additionally explore the primary parts of cobalt(III)-hydroperoxo particulars, the exploration group utilized computational science reproductions, which influence the force of PC registration to break down substance peculiarities. These reproductions featured the effect of changes in the blend of molecules on the construction of cobalt (III)-hydroperoxo particulars, reaffirming the essential job of basicity.
Teacher Cho expressed, “This exploration reveals the hidden components of metal-dynamic oxygen species in enacting nitrile, filling in as an establishment for the future improvement of impetuses equipped for initiating nitrile.”
More information: Yeongjin Son et al, Mechanistic Insights into Nitrile Activation by Cobalt(III)–Hydroperoxo Intermediates: The Influence of Ligand Basicity, JACS Au (2023). DOI: 10.1021/jacsau.3c00532