Name a natural capability, and proteins called integrins are likely engaged with it.
Together, the 24 individuals from the integrin family permit cells to join with each other and with the grid that encompasses them. They assist cells with choosing what to become, where to go, how to respond to their surroundings, and when to develop, gap, or pass on.
Integrins’ universality and flexibility likewise imply that while cells bearing them turn out badly, these proteins can add to a scope of illnesses, from immune system infections to disease.
The FDA has up to this point endorsed six medications that lessen the action of explicit integrins to treat diseases like various sclerosis and ulcerative colitis and to forestall blood clumps from forming. To the failure of researchers, specialists, and patients, nonetheless, other promising applicants have bombed in clinical preliminaries and reduced integrins’ true capacity as treatment targets.
“The same water-harvesting design approach has already been extended to another integrin, and structural data suggests that researchers can build medications to target additional members of the integrin family to cure disorders that cause considerable pain,”
Springer, who is a member of the Program in Cellular and Molecular Medicine at Boston Children’s.
New work driven by scientists at Harvard Clinical School and Boston Kids’ Clinic reveals a justification for the disappointments—and offers a likely arrangement.
Investigating an integrin engaged with blood thickening, Timothy Springer, the Latham Family Teacher of Natural Science and Sub-atomic Pharmacology at HMS and Boston Kids’, and partners found that bombed drugs for two unique integrins incidentally urge the integrins to open up into their “on” position, possibly driving integrin action as opposed to controlling it.
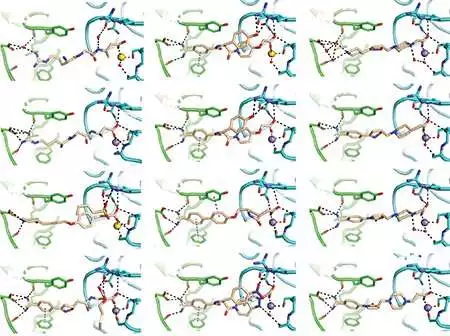
The group made a portion of its disclosures utilizing X-beam crystallography, a careful strategy for determining the sub-atomic designs of proteins. These are a portion of the subsequent charts of medications bound to the coagulation-related integrin. Fu-Yang Lin, Jing Li, Yonghua Xie, et al., Cell
The group uncovered that in its shut or “off” position, the integrin contains a water particle held set up by a progression of compound bonds. The integrin launches the water atom when enacted.
When they realized what was going on, the scientists had the option to engineer integrin blockers that cajoled the thickening protein into its “off” position by holding the water particle set up with a nitrogen iota.
Further tests implied that water atoms assume a similar role in other integrins, showing that the group’s system could work more extensively.
The discoveries, published in the journal Cell on Sept. 15, provide a more clear path for drug improvement and extend scientists’ understanding of how integrins work typically.
“A similar water-tackling plan rule has previously been reached out to another integrin, and primary data proposes that scientists can configure medications to target further individuals from the integrin family to treat illnesses that cause extraordinary misery,” said Springer, who is an individual from the Program in Cell and Sub-atomic Medication at Boston Children’s.
“It’s continuously satisfying to deal with a task that is both logically and medicinally significant,” he added.
More information: Fu-Yang Lin et al, A general chemical principle for creating closure-stabilizing integrin inhibitors, Cell (2022). DOI: 10.1016/j.cell.2022.08.008
Journal information: Cell