A highly active and selective catalytic material that can efficiently convert methane, a potent greenhouse gas, into formaldehyde, an essential chemical, in a waste-free manner has been developed jointly by Professor Zhengxiao Guo from the Department of Chemistry at the University of Hong Kong (HKU) and Professor Junwang Tang from the Department of Chemical Engineering at Tsinghua University.
This novel material, which is made from tungsten trioxide (WO3) catalyst, has a dual active site with atomic species of copper and tungsten that work together to make sure the conversion process is efficient and selective. Under visible light, the conversion process can achieve nearly 100 percent selectivity, avoiding undesirable byproducts and increasing efficiency, making it an eco-friendly alternative to current production methods. The results were recently published in Nature Communications.
“Solar methane conversion is highly desirable for low-carbon and high-value-added chemical synthesis. Product selection and manufacturing efficiency, on the other hand, are critical to success.”
Professor Zhengxiao Guo, one of the corresponding authors of the paper.
Change the status quo: Methane change
Methane, the primary part of petroleum gas, is a generally utilized carbon hotspot for various synthetics. Despite this, it is also a potent greenhouse gas, with the potential to cause more than 70 times as much warming as carbon dioxide. As a result, the catalytic conversion of methane—the process of transforming methane into other chemicals—offers a significant chance to achieve net-zero energy and chemical supplies while simultaneously dealing with environmental issues.
Methane, on the other hand, is an extremely stable molecule that cannot be activated, even in mild or ambient conditions. Since selective activation of the intermolecular carbon-hydrogen bond is one of the most elusive “holy grails” in catalysis, achieving high activity and selectivity in methane conversion is a significant challenge.
Formaldehyde, then again, is a high-volume product substance with a market worth of USD 8 billion, extending at a yearly growth rate (CAGR) of 5.7%. It is a valuable precursor for melamine, urea-formaldehyde, and phenolic resins, among other things, and it is utilized in products for the automotive, medical, commercial, aviation, household, and commercial markets. Formaldehyde is additionally securely utilized in the assembly of immunizations against infective medications and hard-to-swallow pills. It is currently produced by oxidation-dehydrogenation of methanol using silver or metal-oxide catalysts at high reactor temperatures of more than 500°C–600°C, resulting in significant energy losses and emissions of carbon dioxide.
Harnessing sunlight to convert methane
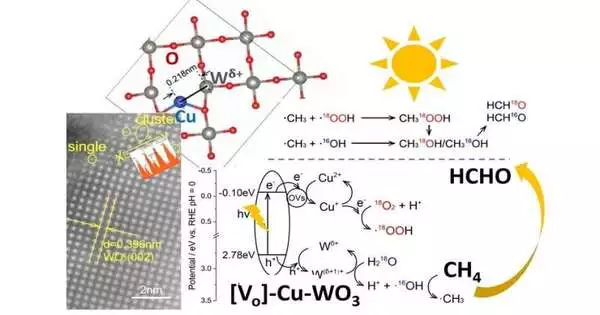
In their study, the team discovered a novel method for converting methane gas into formaldehyde using sunlight. Over tungsten oxide, they discovered that a synergistic mixture of atomically dispersed copper and partially reduced tungsten species produced excellent photocatalytic methane conversion to formaldehyde under ambient visible light. With a turnover frequency of 8.5 106 mol (HCHO) g-1 (cocatalyst) h-1, the process outperformed previously reported photocatalysts in terms of selectivity and conversion efficiency.
They discovered through mechanistic analysis that the tungsten helped activate the methane gas, and the copper helped move electrons around and produce reactive molecular species. Reactive hydroperoxyl radicals (HOO) were produced as a result of copper’s role as electron acceptors and its promotion of photo-induced electron transfer from the conduction band to dioxygen. In the interim, the nearby tungsten iota that had a fractional positive charge worked as opening acceptors. The favored adsorption and enactment site of water delivered hydroxyl extremists and actually actuated methane for methyl revolutionaries. The conversion process’s overall efficiency and selectivity are greatly enhanced by the synergy of the adjacent dual active sites.
This tracking down makes ready for additional innovative work of new photographic impetuses for different substance transformations, advancing more reasonable and productive cycles in the synthetic business.
“Both low-carbon and high-value-added chemical syntheses benefit greatly from solar methane conversion. However, the key to success is product selectivity and production efficiency. This requires an inside-out comprehension of the transformation system, a cautious plan for the impetus, and corresponding methods to affirm its exhibition — a decent instance of multidisciplinary undertakings that require solid cooperative devotion. That is precisely the thing the group has figured out how to do — with much worth added result,” said Teacher Zhengxiao Guo, one of the relating creators of the paper.
More information: Lei Luo et al, Nearly 100% selective and visible-light-driven methane conversion to formaldehyde via. single-atom Cu and Wδ+, Nature Communications (2023). DOI: 10.1038/s41467-023-38334-7