As the utilization of electric and hybrid vehicles expands in numerous nations around the world, the improvement of protected and better-performing battery advances turns out to be progressively essential. Most recently, engineers have been attempting to build the wellbeing and energy limit of batteries while additionally guaranteeing their versatility and dialing back their corruption after some time.
The battery advancements that could uphold the requests of the gadget business incorporate battery-powered multivalent metal batteries (i.e., batteries utilizing multivalent particles) in view of anode materials with low-decrease possibilities, like magnesium (Mg) and calcium (Ca). These batteries could display high energy densities whenever created, utilizing the right blend of anodes, cathodes, and electrolytes.
As of late, studies have recognized different smart anode materials for these batteries. A significant number of the proposed electrolytes, then again, are either hard to source or depend on complex combination processes, which makes them challenging to manufacture for a huge scope.
“A fully dissociated magnesium organoborate electrolyte allows for high current endurance and improved electrochemical kinetics, while a Ca organoborate electrolyte with strong coordination and B–H inclusion provides a stable solid–electrolyte interphase with high coulombic efficiency. These adjustments can be made logically to fine-tune anion participation in the primary solvation shell.”
Siyuang Li, Jiahui Zhang, and their colleagues wrote in their paper.
Specialists at Zhejiang College, the ZJU-Hangzhou Worldwide Logical and Mechanical Development Place, and Dalian College of Innovation as of late presented a new, all-inclusive technique to acknowledge profoundly performing and versatile electrolytes for multivalent metal batteries. Their proposed methodology, illustrated in a paper in Nature Energy, could assist with conceiving reversible and more reasonable electrolyte frameworks, which could demonstrate importance for cutting-edge battery advances.
“Superior execution and cost-efficient electrolyte frameworks are pursued for high-energy-thickness multivalent metal batteries,” Siyuang Li, Jiahui Zhang, and their partners wrote in their paper.
“Notwithstanding, the costly forerunner and complex union cycle prevent investigation of cathode terminal/electrolyte points of interaction and solvation structures. We fostered an all-inclusive cation substitution strategy to get ready minimal expense, high-reversibility magnesium and calcium electrolytes got from a zinc organoborate solvation structure.”
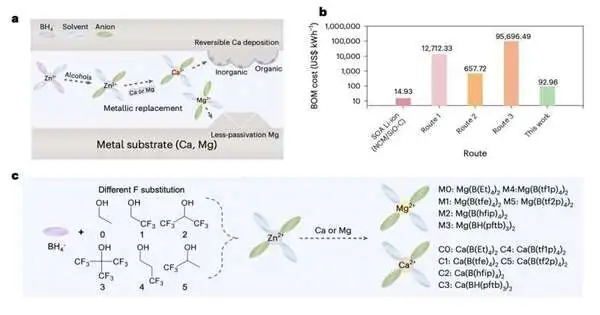
The strategy presented by this exploration group traverses different advances. The scientists, first and foremost, provoked a compound response between a reasonable and effectively feasible Zn(BH4)2 forerunner and various fluoroalcohols, delivering objective anions with different stretched chains.
In this way, these anion solvates responded with minimal-cost metal foils with a higher metal movement to create target solvation structures. To smother the persistent deterioration of solvents and keep up with stable battery cycling, the scientists proposed the development of a passivation layer in light of two kinds of Ca solvates.
“By judiciously changing the antecedent chain length and F-replacement degree, we can tweak anion support in the essential solvation shell,” the scientists made sense of in their paper. “A totally separated Mg organoborate electrolyte empowers high current perseverance and upgraded electrochemical energy, while the Ca organoborate electrolyte with solid coordination/B-H consideration offers a steady, strong electrolyte interphase with high coulombic proficiency.”
The specialists have up to this point utilized their technique to make a 53.4 Wh kg−1 high-stacking battery model in view of Mg/S, which contained a 30 μm Mg anode, a low electrolyte/sulfur proportion (E/S = 5.58 μl mg−1), and a changed separator/interlayer. In the beginning tests, the battery model accomplished promising outcomes, featuring the commitment of this way to deal with making good and minimal-cost electrolytes for multivalent metal batteries.
Later on, the strategy presented in this paper could prepare for the formation of different reversible electrolyte frameworks that depend on additional reasonable materials and more straightforward handling systems. These electrolytes could be utilized to make adaptable and safe multivalent metal batteries with higher energy densities.
More information: Siyuan Li et al. Cation replacement method enables high-performance electrolytes for multivalent metal batteries, Nature Energy (2024). DOI: 10.1038/s41560-023-01439-w