Warfarin, a broadly utilized blood thinner, seems to have strong anti-cancer growth properties, as indicated by a concentrate by Columbia College specialists. The review, conducted in human cells and in mice, found that warfarin prevents cancers from slowing down a fall-to-pieces component that cells start when they distinguish transformations or different irregularities.
“Our discoveries recommend that warfarin, which is now supported by the FDA, could be reused to treat various diseases, including pancreatic malignant growth,” says concentration on pioneer Wei Gu, Ph.D., the Abraham and Mildred Goldstein Teacher of Pathology and Cell Science (in the Foundation for Disease Hereditary Qualities) at Columbia College Vagelos School of Doctors and Specialists.
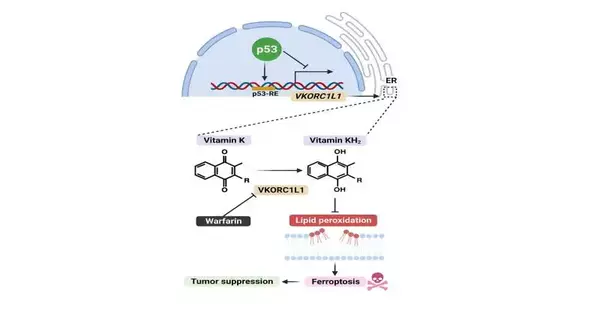
The review is named “Guideline of VKORC1L1 is basic for p53-intervened cancer concealment through vitamin K digestion,” and it was distributed July 18 in Cell Digestion. Postdoctoral analyst researchers Xin Yang, Ph.D., and Zhe Wang, Ph.D., contributed similarly as first creators.
“Our results indicate that warfarin, which has already received FDA approval, may be used to treat a number of malignancies, including pancreatic cancer.”
Study leader Wei Gu, Ph.D., the Abraham and Mildred Goldstein Professor of Pathology & Cell Biology .
Passing by ferroptosis
The warfarin disclosure was a startling finding from a review intended to reveal sub-atomic cycles that manage ferroptosis, a cell demise system as of late found by Columbia physicist Brent Stockwell, Ph.D., an academic partner in the Divisions of Natural Sciences and Science, and different researchers.
Malignant growth analysts are invigorated by outfitting ferroptosis—so-named on the grounds that it expects iron to work—tto kill disease cells. Drugs that prompt ferroptosis might be especially helpful for tumors that evade current medicines.
To become familiar with how ferroptosis is controlled in the cell, Gu, Stockwell, and their partners performed hereditary screens on human melanoma cells to recognize qualities that contribute to ferroptosis. True to form, the screens distinguished a few recently known ferroptosis qualities, yet at the same time, another one stuck out: VKORC1L1.
In lab tests, the scientists observed that VKORC1L1 is a powerful inhibitor of ferroptosis, and deficiency of VKORC1L1 sharpens cells to ferroptotic cell demise.
VKORC1L1 levels likewise have clinical results; an investigation of human disease information then, at that point, uncovered: Patients with low degrees of VKORC1L1 action commonly lived longer than patients with more elevated levels.
Warfarin advances ferroptosis in malignant growth cells.
Warfarin (likewise known by the trademark coumadin) was first endorsed for clinical use in 1954. It has since turned into a pillar treatment for preventing blood clusters, which can cause stroke, cardiovascular failure, or pneumonic embolism.
Warfarin is likewise a known VKORC1L1 inhibitor, so the specialists investigated its true capacity as a malignant growth drug. They tracked down that warfarin, by lessening VKORC1L1 action, sharpened human pancreatic disease cells to ferroptosis and emphatically subdued growth development in a mouse model of pancreatic disease.
Information from different examinations additionally supports the possibility that warfarin has potential against disease. Warfarin and different anticoagulants are ordinarily given to disease patients who are at increased risk for blood clusters. As of late, examiners have seen that pancreatic, gastric, and colorectal malignant growth patients who got warfarin endured fundamentally longer than those taking different anticoagulants.
“Since warfarin has been widely utilized in the facility in malignant growth patients, we figure warfarin could be tried soon as an anticancer medication, especially for cancers with elevated degrees of VKORC1L1 articulation,” Gu says.
That might reach beyond pancreatic and gastric tumors to numerous different sorts, Gu adds. The specialists likewise observed that VKORC1L1 is an immediate objective of p53, a notable growth silencer quality that is transformed in more than 50% of all tumors.
More information: Xin Yang et al, Regulation of VKORC1L1 is critical for p53-mediated tumor suppression through vitamin K metabolism, Cell Metabolism (2023). DOI: 10.1016/j.cmet.2023.06.014