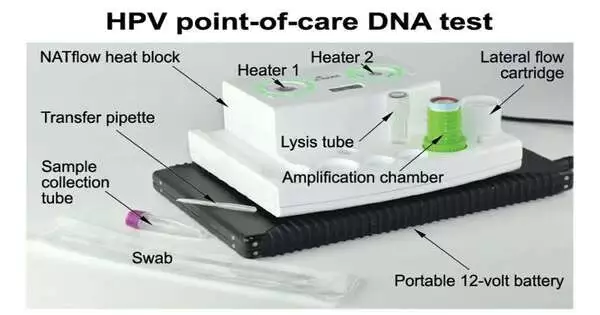
Bioengineers at Rice University have demonstrated a low-cost DNA test for HPV infections that can be used at the point of care. This test has the potential to make cervical cancer screening more accessible in low- and middle-income countries, where the disease kills more than 300,000 women every year.
A group of viruses known as HPV infects nearly everyone at some point in their lives, frequently without causing symptoms. However, more than a dozen different kinds of HPV have the potential to cause persistent infections that eventually lead to cervical cancer. Cervical cancer can be avoided and treated effectively if it is caught early.
A DNA testing platform that combines two technologies—isothermal DNA amplification and lateral flow detection—in a way that greatly simplifies the requirements for testing equipment was developed by nine engineers from Professor Rebecca Richards-Kortum’s laboratory over the course of more than two years.
“At this point, it’s really a matter of access, and that’s one of the reasons this study is exciting from a global health standpoint. It displays a testing procedure that might be integrated with point-of-care diagnostic and treatment technology to allow women who have never had access to be checked and treated in settings such as a tiny clinic or a mobile diagnostic van.”
First author Kathryn Kundrod, a cancer prevention fellow at the National Cancer Institute.
Richards-Kortum’s team and co-authors from the National Cancer Institute, the Mozambique Ministry of Health, Baylor College of Medicine, and the University of Texas MD Anderson Cancer Center demonstrated that the platform could produce results that are clinically relevant on samples collected at both clinical sites in the United States and clinical field sites in Mozambique in a study that was published this week in Science Translational Medicine.
Kathryn Kundrod, the study’s first author and a cancer prevention fellow at the National Cancer Institute and senior advisor for cancer moonshot policy coordination at the White House Office of Science and Technology Policy, stated, “We know what we need to do to prevent cervical cancer.” At this point, it really comes down to access, which is one reason why this study is exciting from a global health perspective. It demonstrates a testing procedure that could possibly be combined with point-of-care diagnostic and treatment technologies to enable women who have never had access to screening and treatment in a single visit in settings such as a mobile diagnostic van or a small clinic.”
The researchers demonstrated in the study that their six-step test for HPV16 and HPV18—two types that account for approximately 70% of cervical cancer—provided results in 45 minutes and required only two pieces of equipment. A small centrifuge, which costs about $500, is widely available. The researchers were able to use disposable cartridges to avoid false positives caused by workspace contamination, a major obstacle for point-of-care molecular testing, thanks to the other heater, NATflow, which was designed specifically for this purpose and has two chambers.
Richards-Kortum, the Rice360 Institute for Global Health Technologies’ founding director and Malcolm Gillis University Professor of Bioengineering, stated, “The vast majority of disease detected through screening is precancerous, before the point at which people have cancer.” Because of this, screening programs are so successful. Cervical cancer is very rare in people who have regular screenings. People who have never been screened before or who only get screened once or twice a year are most at risk. As a result, it is critical to address disparities and consider novel screening, diagnosis, and treatment methods.”

Kathryn Kundrod ’20, Rebecca Richards-Kortum, and Mary Natoli ’20 are pictured here in Richards-Kortum’s Rice University laboratory in March 2020, from left to right. A new study co-authored by Kundrod, Natoli, and Richards-Kortum demonstrated the efficacy of a low-cost, point-of-care DNA test for HPV infections. This could make it possible to increase the number of cervical cancer screenings in low- and middle-income nations, where the disease kills more than 300,000 women annually. Credit: Jade Boyd/Rice University.
Kundrod, a former Rice student and postdoctoral researcher who led the day-to-day development of the HPV test and the co-development of the platform with NATflow manufacturer Axxin of Victoria, Australia, will earn her Ph.D. from Rice in 2020. According to Kundrod, if the NATflow platform and test cartridges were manufactured on a large scale, each dual-chamber heater would cost approximately $500, while each test cartridge would cost less than $5.
She remarked, “The platform is the other thing that makes this exciting, because it can be easily adapted for DNA tests for other diseases.” DNA-based point-of-care tests have struggled greatly with the issue of contamination prevention. This is one of the first platforms to address that, and it is the only one to date to do so in a way that makes it simple to produce all of the pieces using injection molding, which is important from a cost standpoint.”
According to Kundrod, the Rice team’s HPV test will not be ready for widespread use until researchers modify it to detect additional HPV types that cause cancer and carry out additional clinical tests. She stated that DNA testing is the most effective method for screening for HPV infections, and studies have consistently demonstrated that HPV screening is the most effective method for preventing cervical cancer.
Richards-Kortum stated, “We are committed to continued development and scale in places where cervical cancer screening is most needed.” She expressed gratitude to the entire team for making this work possible.
More information: Kathryn A. Kundrod et al, An integrated isothermal nucleic acid amplification test to detect HPV16 and HPV18 DNA in resource-limited settings, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abn4768