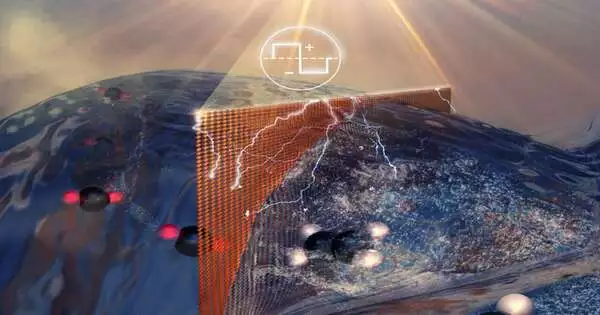
A group of scientists led by Meenesh Singh at the College of Illinois Chicago has found a method for changing over 100 percent of carbon dioxide from modern exhaust into ethylene, a key building block for plastic items.
Their discoveries are distributed in Cell Reports: Actual Science.
While scientists have been investigating the possibility of changing carbon dioxide completely to ethylene for over 10 years, the UIC group’s methodology is quick to accomplish almost 100 percent use of carbon dioxide to create hydrocarbons. Their framework utilizes electrolysis to change caught carbon dioxide gas into high-virility ethylene, with other carbon-based fills and oxygen as results.
The cycle can change up to 6 metric tons of carbon dioxide into 1 metric ton of ethylene, reusing practically all the carbon dioxide caught. Since the framework runs on power, the utilization of sustainable power can make the cycle carbon negative.
As per Singh, his group’s methodology outperforms the net-zero carbon objective of other carbon capture and change advances by really lessening the all-out carbon dioxide yield from industry. “It’s a net negative,” he said. “For each ton of ethylene created, you’re taking 6 tons of CO2 from point sources that generally would be delivered to the air.”
Past efforts to change carbon dioxide into ethylene have depended on reactors that produce ethylene inside the source carbon dioxide outflow stream. In these cases, just 10% of CO2 outflows are commonly converted to ethylene. The ethylene should later be isolated from the carbon dioxide in an energy-serious cycle frequently including petroleum products.
In UIC’s methodology, an electric flow is passed through a cell, a big part of which is loaded up with caught carbon dioxide, the other half with a water-based arrangement. An electric impetus draws charged hydrogen iotas from the water particles into the other portion of the unit isolated by a film, where they join with charged carbon iotas from the carbon dioxide atoms to frame ethylene.
Among man-made synthetics around the world, ethylene ranks third for fossil fuel byproducts after alkali and concrete. Ethylene is used not only to make plastic items for the packaging, rural, and automobile industries, but also to make synthetics used in radiator fluid, clinical sanitizers, and vinyl siding for houses.
Ethylene is normally made in a cycle called steam breaking that requires huge measures of intensity. Breaking produces around 1.5 metric tons of fossil fuel byproducts per ton of ethylene made. Overall, makers produce around 160 million tons of ethylene every year, which brings about in excess of 260 million tons of carbon dioxide outflows around the world.
Notwithstanding ethylene, the UIC researchers had the option to create other carbon-rich items helpful to industry with their electrolysis approach. They also achieved a high sun-based energy change proficiency, converting more than 10% of the energy from the solar chargers directly to carbon item yield. This is well over the cutting edge norm of 2%. For all the ethylene they created, the sun’s based energy change proficiency was around 4%, roughly the same rate as photosynthesis.
More information: Aditya Prajapati et al, CO2-free high-purity ethylene from electroreduction of CO2 with 4% solar-to-ethylene and 10% solar-to-carbon efficiencies, Cell Reports Physical Science (2022). DOI: 10.1016/j.xcrp.2022.101053
Journal information: Cell Reports Physical Science