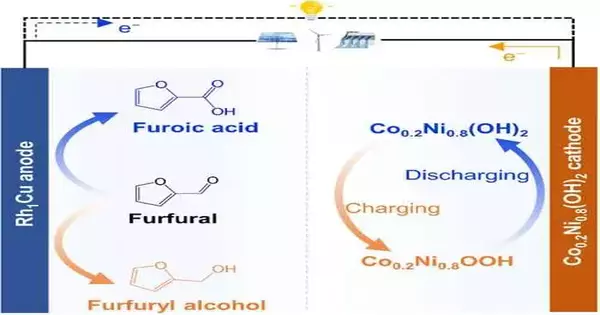
Redox flow batteries use chemicals stored in tanks attached to the electrodes, whereas rechargeable batteries store electricity in their electrode materials. A hybrid cell-based battery system that not only stores and provides electricity but also produces valuable chemicals in a flow system has now been developed by researchers. The biomass-derived molecular furfural is transformed into either furfuryl alcohol or furoic acid by the furfural-nickel hydroxide battery during operation.
Furfural is a small molecule that is made from pentose sugars that are found in agricultural biomass. It is regarded as an important platform chemical from which a number of intermediates can be made for a variety of uses. It tends to be oxidized to furoic acid, corrosive, a food additive, and moderate in the union of drugs and scents. Furfur is transformed into furfuryl alcohol when reduced, which is used as a precursor in drugs, flavors, and resins. Now that Haohong Duan and a group of researchers from Beijing’s Tsinghua University have been successful in obtaining both value-added chemicals while a hybrid flow battery is operating, the battery system’s cost efficiency has increased.
The group’s examination is distributed in the journal Angewandte Chemie Worldwide Version.
Standard rechargeable batteries discharge by feeding electricity into a circuit and storing it in their electrodes when charged. Redox flow batteries, a different kind of battery, store electricity in chemicals. The chemicals go through two states and stay in the battery. Combining the two ideas, the scientists explored the degree to which such batteries can deliver additional synthetics while putting away or giving energy.
A bifunctional metal anode catalyst marked a significant advance. Made of a rhodium-copper single-particle combination, this impetus easily changed electrolyte-containing furfural into furfuryl liquor when the battery was charged, while furoic corrosive was framed as the battery was released. The researchers found a cobalt-doped nickel hydroxide for the cathode that was comparable to the cathode materials found in conventional nickel-zinc or nickel-metal hydride batteries.
A genuine dual-use battery system resulted from this assembly. Four series-connected hybrid batteries were able to run a variety of devices, including smartphones and LED lights, after being charged with a solar cell. During battery cycling, the batteries produced furfuryl alcohol and furoic acid continuously, and a flow system carried these chemicals away.
The creators observed that the new crossover battery is equivalent to various normal batteries concerning energy thickness and power thickness, but it gives both power and value-added synthetic compounds simultaneously. Furfuryl alcohol weighs 0.7 kilograms when stored, and furoic acid weighs 1 kilogram when the system generates 0.5 kWh of power—enough to power a refrigerator for a few hours. Notwithstanding, furfural is persistently taken care of in the framework, and the items should be isolated from the electrolyte.
The group’s half-and-half idea is a stage toward working on the manageability and cost viability of battery-powered batteries, yet there is as yet a need to foster the idea further.
More information: Jing Li et al, Rechargeable Biomass Battery for Electricity Storage/generation and Concurrent Valuable Chemicals Production, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202304852