Adam Brandt, an energy science and ecological designer at Stanford College, has directed a day-to-day existence cycle evaluation of hydrogen creation from regular subsurface gatherings, otherwise called land hydrogen extraction, to decide if the thought is viable. In his paper, distributed in the journal Joule, he sees factors engaged with removing topographical hydrogen, for example, the effect on environmental change and its accessibility for extraction.
As the world keeps on warming (July 2023 is on course to be the most sweltering month at any point recorded), researchers are searching for ways of supplanting ozone-depleting substance-delivering energy sources with those that are more cordial to the climate. One such methodology includes the utilization of hydrogen gas as a fuel source.
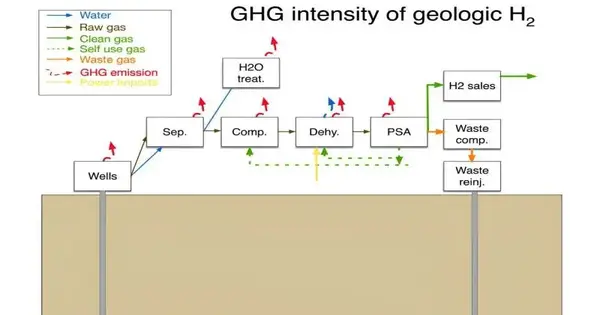
Hydrogen is viewed as a “green” sort of gas since it may deliver water when consumed. Sadly, the most famous means for creating hydrogen include consuming flammable gas, which produces ozone-harming substances. As a result, researchers have been investigating alternative methods for obtaining hydrogen. One such cycle includes separating topographical hydrogen from the beginning; moving toward that has not gotten a lot of consideration as of not long ago. Brandt examines the factors involved in obtaining hydrogen from underground sources in this new endeavor.
Brandt’s analysis centers primarily on whether hydrogen extraction is more or less harmful to the environment. He points out that it is still unclear whether the idea of extracting hydrogen for commercial use is a viable one because it is still so new. For instance, he takes note of the fact that removal activities would prompt some spillage of the gas into the environment. Even though hydrogen is not a greenhouse gas, it reacts with other greenhouse gases to make them last longer.
Another issue is that land hydrogen is seldom unadulterated; more often than not, it is blended in with methane, nitrogen, or different gases, which would all need to be taken out before use. Such handling would require energy, which could prompt ozone-harming substance emanations. Additionally, he makes the observation that researchers have not yet established whether or not there is sufficient geological hydrogen for harvesting.
Brandt comes to the conclusion that, despite the difficulties, it appears that further investigation into the concept of geological hydrogen extraction is required. His analysis revealed that, in its worst case, it would probably not be any worse than “green hydrogen,” which is produced by splitting water molecules.
More information: Adam R. Brandt, Greenhouse gas intensity of natural hydrogen produced from subsurface geologic accumulations, Joule (2023). DOI: 10.1016/j.joule.2023.07.001