Medical experts have devised a method for creating a low-cost, simple-to-use focused ultrasound device that can aid in the opening of the blood-brain barrier for non-invasive treatments and diagnostics.
The development of multi-element arrays for greater control of ultrasonic beam form has paved the way for focusing through highly aberrating material such as the human skull. As a result, the use of transcranial-focused ultrasound for brain therapy has risen quickly. Although effective, such equipment is prohibitively expensive.
Researchers and doctors have been striving to breach the blood-brain barrier (BBB) using focused ultrasound and microbubbles for noninvasive diagnostics as well as to deliver therapies to the brain for cancers and neurodegenerative illnesses. However, the few preclinical research equipment that are now available are costly, large, and lack the precision required for small animal research.
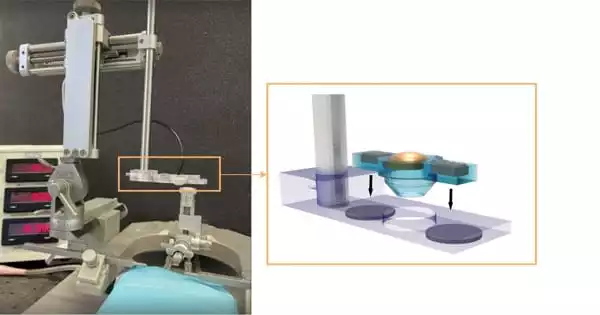
Hong Chen, associate professor of biomedical engineering at the McKelvey School of Engineering and radiation oncology at the School of Medicine at Washington University in St. Louis, and her colleagues have developed a low-cost, simple-to-use, and highly precise focused ultrasound (FUS) device that can be used on small animal models in preclinical research.
We demonstrated that, at the same pressure level, a higher-frequency FUS transducer achieved a small drug delivery volume, improving the spatial precision of BBB opening compared to lower-frequency transducers.
Hong Chen
The FUS transducer, which was built in-house using a 3D printer, costs around $80 to make. It can be combined with a commercially available stereotactic frame to accurately target the brain of a mouse. The research findings were published online in IEEE Transactions on Biomedical Engineering.
According to Chen, the gadget has various advantages, including sub-millimeter targeting accuracy and an adjustable drug-delivery outcome. Furthermore, using higher frequency FUS transducers reduced BBB opening volume and enhanced FUS-BBB opening precision in targeting particular regions in the mouse brain.
“We demonstrated that, at the same pressure level, a higher-frequency FUS transducer achieved a small drug delivery volume, improving the spatial precision of BBB opening compared to lower-frequency transducers,” Chen explained.
High-intensity focused ultrasound (HIFU) is a non-invasive therapeutic approach that use non-ionizing ultrasonic pulses to heat or ablate tissue. HIFU can be used to boost blood or lymph flow or to kill tissue, such as tumors, using heat and mechanical forces. Because of the availability and low cost of ultrasonography, HIFU has been the focus of extensive research and development. The idea of HIFU is that it is a non-invasive, low-cost therapy that can outperform the existing standard of care at the very least.
The team merely needed to connect wires to the electrodes on the elements to make the transducer. The remaining parts were created using a 3D printer. Her team was able to target the exact location they desired in the brain using a stereotactic frame, removing one of the impediments to more widespread adoption of the FUS procedure. Chen’s team has published the design on Github.
“We anticipate that this device may be made by research groups with no ultrasonic experience and employed in a variety of preclinical research applications with minimum training,” Chen added.
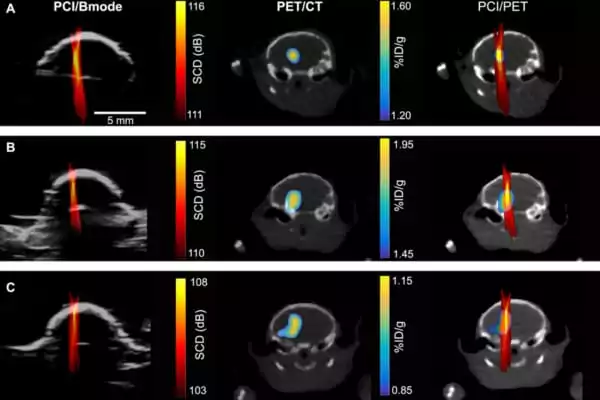
The researchers employed contrast-enhanced MRI to estimate the BBB opening volume at various sonic pressures and then used a model drug to evaluate the drug delivery outcome. The gadget was determined to be extremely safe, with only two mice experiencing microhemorrhages at the highest tested sonic pressures and no tissue damage in the other groups.
Focused ultrasound is a technique that employs ultrasonic energy to target tumors or tissue in the brain. Once identified, the researchers inject microbubbles into the bloodstream, which travel to the targeted region and then rupture, causing microscopic tears in the blood-brain barrier. The ruptures allow medications or biomarkers from tumors to flow through the blood-brain barrier and into the bloodstream. Chen and her colleagues have been honing the approach in preclinical animals for several years.
There is no clear agreement on the distinctions between HIFU and other types of therapeutic ultrasonography. HIFU is commonly used in academic literature to explain the high levels of energy required to kill tissue via ablation or cavitation, but it is also used to describe lower intensity uses such as occupational therapy and physical therapy. In any case, HIFU is utilized to heat tissue deep within the body without the need for an incision. The primary applications are tissue damage, increased perfusion, and physical rehabilitation. Another application of ultrasonography in physiotherapy is the treatment of musculoskeletal problems.
Chen expressed her expectation that this gadget will lower the hurdles to widespread adoption of the FUS technique in the research community.