A clinical preliminary of Public Organizations of Wellbeing has been discovered.The immunizer depended on 88.2% viable at forestalling disease north of a 24-week term, showing interestingly that a monoclonal neutralizer can forestall jungle fever contamination in an endemic locale. These discoveries were distributed today in The New Britain Diary of Medication and introduced at the American Culture of Tropical Medication and Cleanliness 2022 Yearly Gathering in Seattle.
“We want to grow the armory of accessible mediations to forestall jungle fever contamination and speed up endeavors to kill the illness,” said Anthony S. Fauci, M.D., head of the Public Foundation of Sensitivity and Irresistible Illnesses (NIAID), part of NIH. “These review results propose that a monoclonal immunizer might actually supplement different measures to safeguard voyagers and weak gatherings like babies, kids, and pregnant ladies from occasional jungle fever and assist with killing intestinal sickness from characterized geological regions.”
The preliminary was driven by Peter D. Crompton, M.D., M.P.H., and Kassoum Kayentao, M.D., M.P.H., Ph.D. Dr. Crompton is head of the Jungle Fever Disease Science and Resistance Area in the NIAID Lab of Immunogenetics, and Dr. Kayentao is a teacher at the College of Sciences, Methods, and Innovations (USTTB) in Bamako, Mali.
“These preliminary field results demonstrating that a monoclonal antibody safely provides high-level protection against intense malaria transmission in healthy adults pave the way for future research to determine whether such an intervention can prevent malaria infection in infants, children, and pregnant women,”
Robert A. Seder, M.D.,
An expected 241 million instances of jungle fever happened overall in 2020, as per the World Wellbeing Association (WHO), bringing about an expected 627,000 deaths, generally in kids in sub-Saharan Africa. These cases affect in excess of 11 million pregnant people in Africa, bringing about an expected 819,000 babies with low birthweight and hence at increased risk for disease and demise.
The main jungle fever immunization presently suggested by WHO, called RTS,S (Mosquirix), gives halfway security against clinical jungle fever during the early long stretches of life when given to kids aged 5 to 17 months in four dosages over a 20-month time span. Different medications, including minor substance enhancers that effectively prevent jungle fever disease, are also available for babies and small children, as well as travelers.The necessity for regular dosing of these medications can restrict adherence nonetheless, and the rise of medication opposition may likewise restrict their value. Hence, there is a dire requirement for new, effective, rarely dosed mediations that securely give solid assurance against jungle fever disease.
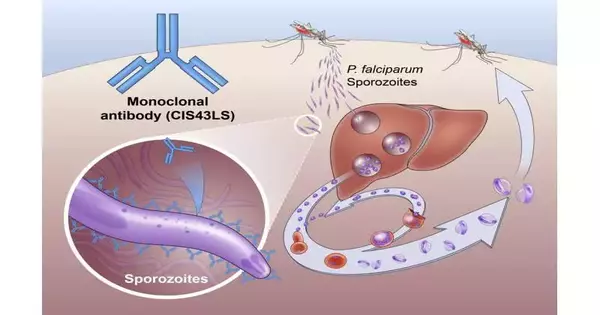
Jungle fever is brought about by Plasmodium parasites, which are transmitted to individuals through the chomp of a tainted mosquito. The mosquito infuses the parasites in a structure called sporozoites into the skin and circulation system. These travel to the liver, where they mature and increase. Then the adult parasite spreads all throughout the body by means of the circulatory system to cause disease. Plasmodium falciparum is the Plasmodium species that is probably going to bring about extreme jungle fever diseases, which — while perhaps not quickly treated — may prompt demise.
The Stage 2 NIAID-USTTB preliminary assessed the security and viability of a one-time, intravenous mixture of a monoclonal immunizer called CIS43LS. This immunizer was recently shown to kill the sporozoites of P. falciparum in the skin and blood before they could taint liver cells. Scientists led by Robert A. Seder, M.D., isolated a naturally occurring type of this immunizer from the blood of a worker receiving an investigational jungle fever immunization and then changed the neutralizer to lengthen the time it remained in the circulatory system.Dr. Seder is the acting chief clinical official and acting partner head of the NIAID Antibody Exploration Center (VRC) and head of the VRC’s Cell Immunology Area.
The review group for the Stage 2 preliminary enlisted 369 sound, non-pregnant grown-ups aged 18 to 55 years in the rustic networks of Kalifabougou and Torodo in Mali, where serious P. falciparum transmission commonly happens from July through December every year.
The initial segment of the preliminary survey surveyed the security of three unique dosages of CIS43LS — 5 milligrams for each kilogram of body weight; 10 mg/kg; and 40 mg/kg — managed by intravenous mixture in 18 review members, with six members for every portion level. The review group followed these members for a long time and found the immunizer mixtures were protected and very much endured.
The second piece of the preliminary survey examined the viability of two unique dosages of CIS43LS contrasted with a fake treatment. 300 and thirty members were relegated indiscriminately to getting either 10 mg/kg of the immunizer, 40 mg/kg, or a fake treatment by intravenous mixture. Nobody realized who was allotted to which bunch for the rest of the preliminary. The review group followed these people for a long time, testing their blood for P. falciparum week by week for the initial 28 days and like clockwork from there on. Any member who created suggestive jungle fever during the prelim got standard treatment from the review group.
The agents examined the adequacy of CIS43LS in two different ways. In view of the possibility of first P. falciparum disease over the 24-week concentrate on period, the high portion (40 mg/kg) of CIS43LS was 88.2% viable at forestalling contamination and the lower portion (10 mg/kg) was 75% powerful. An examination of the extent of members tainted with P. falciparum whenever over the 24-week concentrate on period found the high portion was 76.7% at forestalling disease and the lower portion was 54.2% viable.
“These first field results show that a monoclonal immunizer securely gives an undeniable level of security against serious jungle fever transmission in sound grown-ups” prepare for additional examinations to decide whether such a mediation can forestall jungle fever disease in babies, kids, and pregnant ladies,” Dr. Seder said. “We trust monoclonal antibodies will change jungle fever avoidance in endemic areas.”
Dr. Seder and partners have fostered a second antimalarial monoclonal immunizer, L9LS, that is considerably stronger than CIS43LS and hence can be managed in a more modest portion as an infusion under the skin (subcutaneously), as opposed to by intravenous mixture. A beginning stage NIAID preliminary of L9LS in the US observed that the immunizer protected and forestalled jungle fever disease for 21 days in 15 out of 17 sound grown-ups presented to P. falciparum in a painstakingly controlled setting. Two bigger, NIAID-supported Stage 2 preliminary surveys surveying the security and adequacy of L9LS in babies, kids, and grown-ups are in progress in Mali and Kenya.
More information: Kassoum Kayentao. Testing the safety and efficacy of anti-malaria monoclonal antibodies in African adults and children. Session 41—Progress in the discovery and clinical development of anti-malaria monoclonal antibodies. ASTMH 2022 Annual Meeting, Seattle. Monday, Oct. 31, 2022. 5:40 pm Pacific Time.
Kassoum Kayentao et al, Safety and efficacy of a monoclonal antibody against malaria in Mali. The New England Journal of Medicine DOI: 10.1056/NEJMoa2206966 (2022).