Another sort of immunization gives security against an assortment of SARS-like betacoronaviruses, including SARS-CoV-2 variations, in mice and monkeys, as per a review driven by scientists in the lab of Caltech’s Pamela Bjorkman, the David Baltimore Professor of Biology and Bioengineering.
Betacoronaviruses, including those that caused the SARS, MERS, and COVID-19 pandemics, are a subset of COVIDs that taint people and creatures. The immunization works by giving the safe framework bits of the spike proteins from SARS-CoV-2 and seven other SARS-like betacoronaviruses, joined to a protein nanoparticle structure, to incite the creation of a wide range of cross-receptive antibodies. Rather, when immunized with this alleged mosaic nanoparticle, animal models were protected from an additional COVID, SARS-CoV, that was not addressed in the nanoparticle immunization.
“Creatures immunized with the mosaic-8 nanoparticles evoked antibodies that perceive basically every SARS-like betacoronavirus strain we assessed,” says Caltech postdoctoral researcher Alexander Cohen (Ph.D. ’21), co-first creator of the new review. “A portion of these infections could be connected with the strain that causes the following SARS-like betacoronavirus episode, so what we truly need would be something that objectives this ongoing gathering of infections.” We accept we have that. “
“Animals immunized with mosaic-8 nanoparticles generated antibodies that recognized nearly every SARS-like betacoronavirus strain we tested. Because some of these viruses may be connected to the strain that causes the next SARS-like betacoronavirus outbreak, we really need something that targets this specific group of viruses. That, we believe, we have.”
Caltech postdoctoral scholar Alexander Cohen
The examination shows up in a paper in the journal Science on July 5.
Bjorkman, who is likewise a Merkin Institute Professor and boss for Biology and Biological Engineering, says “SARS-CoV-2 has substantiated itself fit for making new variations that could delay the worldwide COVID-19 pandemic,” adds Bjorkman. Furthermore, the spread of three betacoronaviruses — SARS-CoV, MERS-CoV, and SARS-CoV-2 — into humans from animals in the last 20 years demonstrates the need for highly defensive antibodies.”
Such wide security is required, Bjorkman says, “since we can’t foresee which infection or infections among the huge numbers of creatures will advance in the future to taint people to cause another plague or pandemic.” What we’re attempting to do is make an across-the-board immunization defensive against SARS-like betacoronaviruses, paying little mind to which creature infections could develop to permit human disease and spread. This kind of antibody would likewise safeguard against current and future SARS-CoV-2 variations without the requirement for refreshing. “
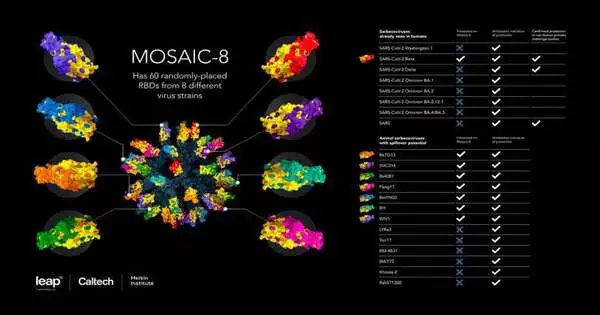
How it functions An immunization made out of spike spaces from eight unique SARS-like Covids
The innovation to join bits of an infection to protein nanoparticles was first grown by partners at the University of Oxford. The premise of the innovation is a small enclosure-like design (a “nanoparticle”) comprised of proteins designed to have “tacky” limbs on their surface, whereupon scientists can join labeled viral proteins. These nanoparticles can be ready to show bits of one infection (“homotypic” nanoparticles) or bits of a few different infections (“mosaic” nanoparticles). When infused into a creature, the nanoparticle immunization presents these viral parts to the safe framework. This incites the creation of antibodies, safe framework proteins that perceive and fend off unambiguous microbes, as well as cell resistant reactions, including T lymphocytes and inborn invulnerable cells.
In this review, the scientists picked eight different SARS-like betacoronaviruses—including SARS-CoV-2, the infection that has caused the COVID-19 pandemic, alongside seven related creature infections that could possibly begin a pandemic in people—and joined parts from those eight infections onto the nanoparticle platform. The group picked explicit parts of the viral designs, called receptor-restriction spaces (RBDs), that are essential for COVIDs to enter human cells. Truth be told, human antibodies that kill COVIDs basically focus on the infection’s RBDs.
The thought is that such an immunization could prompt the body to create antibodies that extensively perceive SARS-like betacoronaviruses to fend off variations notwithstanding those introduced on the nanoparticle by focusing on normal qualities of viral RBDs. This plan comes from the possibility that the variety and actual plan of RBDs on the nanoparticle will concentrate the safe reaction toward parts of the RBD that are shared by the whole SARS group of Covids, hence accomplishing resistance to all. The information detailed in Science today shows the likely adequacy of this methodology.
Planning tests to gauge the antibody’s security in mice
The subsequent antibody (here named mosaic-8) is made out of RBDs from eight Covids. Past tests driven by the Bjorkman lab showed that mosaic-8 prompts mice to create antibodies that respond to an assortment of COVIDs in a lab dish. The new review was meant to work from this exploration to check whether immunization with the mosaic-8 antibody could prompt defensive antibodies in a living creature upon challenge (as such, disease) with SARS-CoV-2 or SARS-CoV.
The group meant to look at how much security against disease was given by a nanoparticle shrouded in various COVID parts (mosaic-8) versus a nanoparticle canvassed in just pieces of SARS-CoV-2 (a “homotypic” nanoparticle).
The group conducted three arrangements of tests on mice. In one, the control, they immunized mice with simply the exposed nanoparticle confine structure with no infection parts joined. A second gathering of mice was infused with a homotypic nanoparticle shrouded exclusively in SARS-CoV-2 RBDs, and a third gathering was infused with mosaic-8 nanoparticles. One trial objective was to check whether vaccination with mosaic-8 would safeguard the creatures against SARS-CoV-2 similarly to the homotypic SARS-CoV-2-inoculated creatures; a subsequent objective was to assess security from a supposed “bungled infection”—one that was not addressed by a RBD on the mosaic-8 nanoparticle.
Quite deliberately, the eight types of COVID covering the mosaic nanoparticle deliberately excluded SARS-CoV, the infection that caused the first SARS pandemic in the mid 2000s. Hence, the group meant to likewise explore the level of security against a test with the first SARS-CoV infection, utilizing it to address an obscure SARS-like betacoronavirus that could pour out into people.
The mice utilized in the tests were hereditarily designed to communicate with the human ACE2 receptor, which is the receptor on human cells that is utilized by SARS-CoV-2 and related infections to acquire sections into cells during disease. In this creature challenge model, unvaccinated mice pass on whenever tainted with a SARS-like betacoronavirus, hence giving a rigid test to assess the potential for security from contamination and illness in people.
A mosaic immunization safeguards mice against a comparable SARS-like betacoronavirus.
True to form, mice immunized with the exposed nanoparticle structure died when tainted with SARS-CoV or SARS-CoV-2. Mice that were immunized with a homotypic nanoparticle just covered in SARS-CoV-2 RBDs were safeguarded against SARS-CoV-2 disease yet passed on upon openness to SARS-CoV. These findings imply that current homotypic SARS-CoV-2 nanoparticle antibody candidates being developed elsewhere would be effective against SARS-CoV-2 but may not provide adequate protection against other SARS-like betacoronaviruses circulating in animal supplies or future SARS-CoV-2 variants.
In any case, the mice immunized with mosaic-8 nanoparticles survive both the SARS-CoV-2 and SARS-CoV challenges with no weight loss or other significant pathologies.
Nonhuman primate research likewise affirms the mosaic antibody’s adequacy.
The group then performed comparative test tests in nonhuman primates, this time utilizing the most encouraging antibody applicant, mosaic-8, and looking at the impacts of mosaic-8 immunization versus no inoculation in creature challenge review. When immunized with mosaic-8, the creatures showed almost no discernible disease when presented with SARS-CoV-2 or SARS-CoV, again exhibiting the potential for the mosaic-8 antibody contender to be defensive against flow and future variations of the infection causing the COVID-19 pandemic as well as against expected future viral overflows of SARS-like betacoronaviruses from creatures.
Critically, as a team with virologist Jesse Bloom (Ph.D. ’07) of the Fred Hutchinson Cancer Research Center, the group found that antibodies evoked by mosaic-8 designated the most well-known components of the RBDs across a different arrangement of other SARS-like betacoronaviruses — the supposed “saved” part of the RBD — hence giving proof to the guessed system by which the immunization would be viable against new variations of SARS-CoV-2 or creature SARS-like betacoronaviruses In contrast, homotypic SARS-CoV-2 nanoparticle infusions elicited antibodies against mostly strain-explicit RBD areas, implying that these immunizations would likely protect against SARS-CoV-2 but not against recently emerging variants or potential arising animal infections.
In a following stage, Bjorkman and partners will assess mosaic-8 nanoparticle vaccinations in people in a Phase 1 clinical preliminary upheld by the Coalition for Epidemic Preparedness Initiative (CEPI). To get ready for the clinical preliminary, which will generally enlist individuals who have been immunized as well as recently tainted with SARS-CoV-2, the Bjorkman lab is arranging preclinical creature model tests to look at safe reactions in creatures recently immunized with an ongoing COVID-19 immunization and reactions in creatures that are immunologically gullible as for SARS-CoV-2 disease or inoculation.
“We have discussed the requirement for variety in antibody improvement since the absolute starting point of the pandemic,” says Dr. Richard J. Hatchett, CEO of CEPI. “The advancement shown in the Bjorkman lab study exhibits immense potential for a system that seeks after another immunization program.”
More information: Alexander A. Cohen et al, Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models, Science (2022). DOI: 10.1126/science.abq0839