“Digestion” depicts the body’s compound changes that make the vital materials for development and generally well-being. As another discovery from Scripps Exploration and its medication advancement arm, Calibr, demonstrates, they could also be powerful atoms for treating severe illnesses.
In a review from Metabolites, published in August 2022, scientists utilized novel medication disclosure advances to reveal a metabolite that converts white fat cells (“terrible” fat) to brown fat (“great” fat) cells. This disclosure offers an expected approach to tending to metabolic circumstances like weight, type 2 diabetes, and cardiovascular illness. Furthermore, it addresses the commitment to using this inventive medication discovery strategy to identify an infinite number of potential therapeutics.
“The explanation for why many sorts of particles don’t go to showcase is a result of harmfulness,” says co-senior writer Gary Siuzdak, Ph.D., the ranking executive of the Scripps Place for Metabolomics and teacher of Science, Sub-atomic and Computational Science at Scripps Exploration. “With our innovation, we can take out endogenous metabolites—meaning the ones that the body makes all alone—that can have a similar effect as a medication with fewer aftereffects. The capability of this approach is even proven by the FDA’s new endorsement of Relyvrio, the mix of two endogenous metabolites for the treatment of amyotrophic sidelong sclerosis (ALS). “
Metabolic illnesses are oftentimes brought about by an unevenness in energy homeostasis—as such, when the body takes in more energy than it uses. Therefore, certain helpful methodologies are based on changing over white fat cells (known as adipocytes) into earthy-colored fat cells. At last, white adipocytes store energy and can ultimately bring about metabolic illnesses like weight gain, while brown adipocytes break up this stored energy into heat, thus expanding the body’s energy use and bringing it back into balance.
To reveal a treatment that could invigorate the creation of brown adipocytes, the scientists looked through Calibr’s Rethink drug-reusing assortment — a library of 14,000 realized drug intensifies that have been endorsed by the FDA for different illnesses or have been widely tried for human security. Utilizing high-throughput screening—a robotized drug disclosure strategy for looking through huge pools of data—the researchers checked Rethink for a medication with these particular capacities.
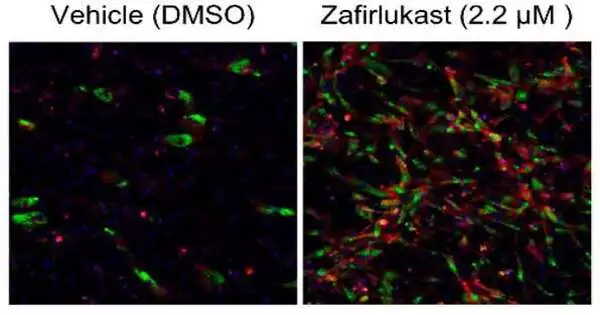
This is the way they revealed zafirlukast, an FDA-endorsed drug utilized for treating asthma. Through a bunch of cell culture tests, they found zafirlukast could turn adipocyte forerunner cells (known as preadipocytes) into predominantly brown adipocytes, as well as convert white adipocytes into brown adipocytes.
While an uplifting find, zafirlukast is harmful when managed at higher dosages, and it wasn’t totally clear how zafirlukast was changing the fat cells. This is the point at which the analysts cooperated with Siuzdak and his group of metabolite specialists.
“We expected to utilize extra devices to separate the synthetics in zafirlukast’s system,” says Kristen Johnson, Ph.D., co-senior creator of the paper and a chief in Translational Medication Revelation Exploration at Calibr. “Outlined another way, might we at any point find a metabolite that has the very useful impact that zafirlukast had, yet without the secondary effects?”
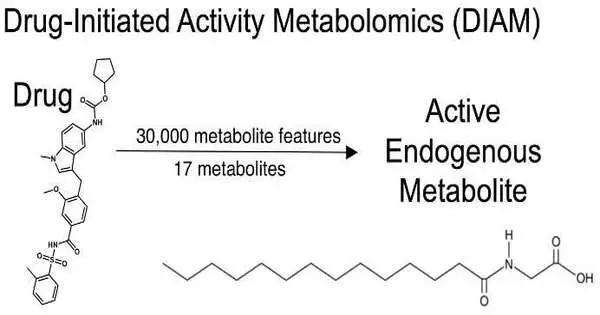
DIAM utilizes advances in, for example, fluid chromatography and mass spectrometry to pool through a great many particles and recognize explicit metabolites. For this situation, the scientists initially decreased 30,000 metabolic elements to only 17 metabolites, and afterward found myristoylglycine—a functioning endogenous metabolite that had the option to change white fat cells completely to brown fat cells, like the medication zafirlukast. Scripps Exploration and Calibration
Siuzdak and his group planned a clever arrangement of tests, known as medication-started action metabolomics (DIAM) screening, to assist with responding to Johnson’s inquiry. DIAM utilizes advances like fluid chromatography (a device that isolates parts in a blend) and mass spectrometry (a logical method that isolates particles by weight and charge) to pool through a great many atoms and recognize explicit metabolites. For this situation, the analysts were scanning through fat tissue for metabolites that could prompt brown adipocyte cell creation.
Upon lessening 30,000 metabolic elements to only 17 metabolites, they found myristoylglycine—an endogenous metabolite that incited the making of brown adipocytes without hurting the cell. Of the great many metabolic elements estimated in the examination, just myristoylglycine had this unique trademark, even among almost primarily indistinguishable metabolites.
“Recognizing myristoylglycine among the great many different particles addresses the force of Siuzdak’s methodology and these innovations,” adds Johnson. “Our discoveries show what happens when a logical science group and a medication discovery group intently team up with one another.”
More information: Carlos Guijas et al, Drug-Initiated Activity Metabolomics Identifies Myristoylglycine as a Potent Endogenous Metabolite for Human Brown Fat Differentiation, Metabolites (2022). DOI: 10.3390/metabo12080749