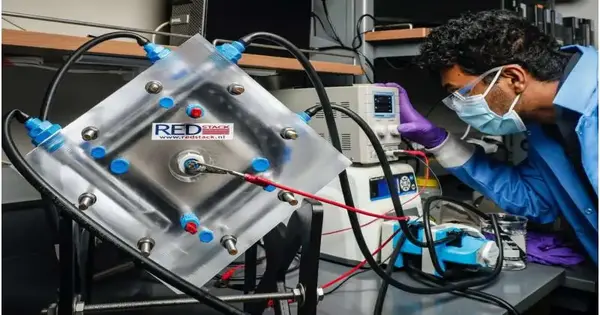
Engineers at the College of Illinois in Chicago have fabricated a machine that catches carbon from vent gas and converts it to ethylene.
Surprisingly, the device integrates a carbon capture framework and an ethylene discussion framework.Besides, the framework runs on power, yet it likewise eliminates more carbon from the climate than it creates, resulting in what researchers call a net-negative impact on fossil fuel byproducts.
Among synthetic materials produced around the world, ethylene ranks third after alkali and concrete in terms of fossil fuel byproducts.Ethylene is utilized not exclusively to make plastic items for bundling, rural, and car ventures, but in addition to creating synthetics utilized in radiator fluid, clinical sanitizers, and vinyl siding for houses, for instance.
The framework and results of the UIC School of Design researchers’ tests are published in an Energy and Natural Science paper titled “Completely Coordinated Electrochemical Framework that Captures CO2 from Vent Gas to Create Valuable Added Synthetics at Surrounding Conditions.”
“This could be a game changer in the quest to make ethylene production more environmentally friendly. The next stage will be to scale up the integrated carbon capture and conversion system to manufacture ethylene at a higher rate—1 kilogram per day—and capture carbon at a rate greater than kilos per day.”
Meenesh Singh, UIC assistant professor in the department of chemical engineering.
“This is the main show of a net-negative, all-electric coordinated framework to catch carbon from poisons and make a profoundly important asset,” said Meenesh Singh, UIC partner teacher in the branch of compound design.
“There is a dire need to foster effective advances for coordinated carbon catch and change to reasonably create net-negative emissions.” As of now, coordinated carbon capture and change frameworks are profoundly energy-serious and work in a broken pattern of carbon dioxide capture and decrease. “Effectively coordinating carbon capture with the change framework eliminates the requirement for transportation and capacity, thereby expanding its energy proficiency.”
The coordinated carbon catch and change framework created at UIC constantly catches carbon dioxide from vent gas to deliver high-value ethylene.
“This is a significant achievement in ethylene decarbonization,” Singh said.
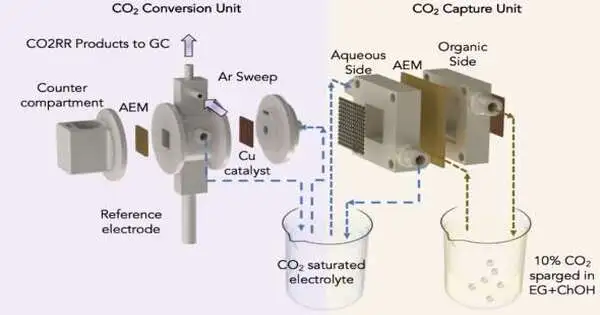
The coordinated framework with movement schematic assisted in dampening the slope CO2 catch and electrochemical CO2 decrease response.
To catch carbon from the air or piping gas, Singh’s lab modified a standard fake leaf framework with cheap materials to incorporate a water slope—aa dry side and a wet side—across an electrically charged film.
On the dry side, a natural dissolvable joins with accessible carbon dioxide to create a grouping of bicarbonate, or baking pop, on the film. As bicarbonate is fabricated, these adversely charged particles are pulled across the film toward a decidedly charged cathode in a water-put-together arrangement with respect to the layer’s wet side. The fluid arrangement breaks up the bicarbonate back into carbon dioxide, so it tends to be delivered and outfitted for CO2 change.
The framework utilizes a measured, stackable plan that permits the framework to be easily increased and decreased.
Singh and his colleagues used a second framework in which an electric flow is passed through a cell to completely convert captured carbon dioxide to ethylene.A large portion of the cell is filled with carbon dioxide captured from a carbon capture framework, while the other half is filled with a water-based arrangement.An electric impetus draws charged hydrogen iotas from the water particles into the other portion of the unit, isolated by a film, where they join with charged carbon iotas from the carbon dioxide atoms to frame ethylene.
The UIC analysts coordinated the two frameworks by reusing the carbon dioxide captured in the carbon change framework.The arrangement’s closed-loop reusing ensures a steady supply of carbon dioxide from pipe gas and its conversion to ethylene.
To test their coordinated framework, the scientists executed a 100-square-centimeter bipolar film electrodialysis unit to catch carbon dioxide from the vent gas and powerfully associate it with the 1-square-centimeter electrolysis cell to create ethylene.
They could test the framework continuously for seven days, 24 hours a day.The framework was not just stable the whole time; it likewise caught carbon at a pace of 24 grams each day and created ethylene at a pace of 188 milligrams each day.
“In the excursion to make ethylene production green, this is a likely forward leap,” Singh said. “Our next stage is proportional to the coordinated carbon catch and change framework to produce ethylene at a faster rate—a rate of 1 kilogram per day—and capture carbon at a faster rate than kilograms per day.”
The co-creators of the review are Aditya Prajapati and Rohan Sartape of UIC, and Miguel Galante, Jiahan Xie, Samuel Leung, Ivan Bessa, Marcio Andrad, Robert Somich, Marcio Reboucas, Gus Hutras, and Nathalia Diniz of Braskem.
More information: Aditya Prajapati et al, Fully-integrated electrochemical system that captures CO2 from flue gas to produce value-added chemicals at ambient conditions, Energy & Environmental Science (2022). DOI: 10.1039/D2EE03396H
Journal information: Energy & Environmental Science