Another computerized reasoning device that deciphers clinical pictures with exceptional lucidity does so in a manner that could permit time-lashed clinicians to commit their consideration regarding basic parts of illness conclusion and picture translation.
The apparatus, called iStar (Gathering Super-Goal Tissue Engineering), was created by scientists at the Perelman Institute of Medication at the College of Pennsylvania, who accept it can help clinicians analyze and better treat malignant growths that could somehow go undetected.
The imaging method gives both profoundly nitty-gritty perspectives on individual cells and a more extensive glance at the full range of how individuals’ qualities work, which would permit specialists and scientists to see disease cells that could somehow have been essentially imperceptible. This device can be utilized to decide if safe edges were accomplished through malignant growth medical procedures and naturally give comments to minute pictures, preparing for sub-atomic sickness findings at that level.
“The power of iStar stems from its advanced techniques, which mimic how a pathologist would examine a tissue sample in reverse. iStar can capture the overarching tissue architecture as well as focus on the minutiae in a tissue image, just as a pathologist detects wider regions and then zooms in on tiny cellular structures.”
Mingyao Li, Ph.D., a professor of Biostatistics and Digital Pathology, was published today in Nature Biotechnology.
A paper on the technique, driven by Daiwei “David” Zhang, Ph.D., an examination partner, and Mingyao Li, Ph.D., a teacher of biostatistics and computerized pathology, was distributed today in Nature Biotechnology.
Li said that iStar can consequently identify the basic enemy of growth-safe developments called “tertiary lymphoid designs,” whose presence corresponds with a patient’s reasonable endurance and positive reaction to immunotherapy, which is many times given for disease and requires high accuracy in persistent choice. This implies, as Li said, that iStar could be a useful asset for figuring out which patients would benefit most from immunotherapy.
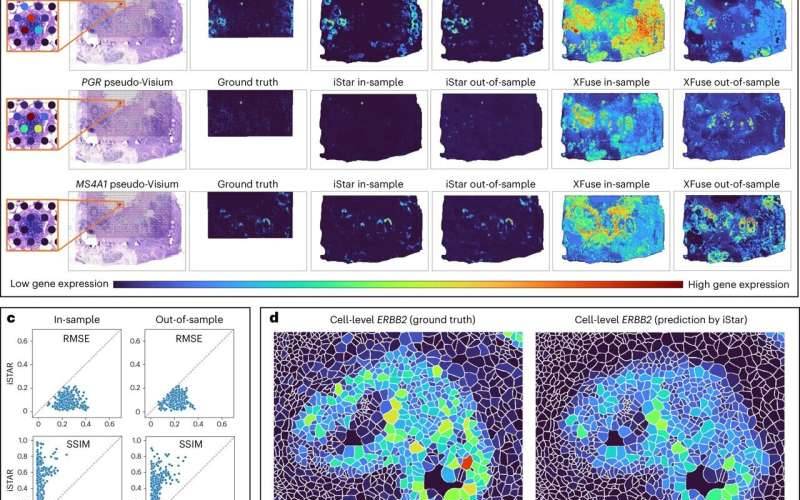
The improvement of iStar was taken on as a component of the field of spatial transcriptomics, a somewhat new field used to plan quality exercises inside the space of tissues. Li and her partners adjusted an AI device called the Various Leveled Vision Transformer and prepared it for standard tissue pictures.
It starts by stalling pictures into various stages, beginning small and searching for fine subtleties, then going up and “getting a handle on more extensive tissue designs,” as indicated by Li. An organization directed by the computer-based intelligence framework inside iStar utilizes the data from the Various Leveled Vision Transformer to then ingest the entirety of that data and apply it to foresee quality exercises, frequently at close single-cell goals.
“The force of iStar comes from its high-level procedures, which reflect, backwardly, how a pathologist would concentrate on a tissue test,” Li made sense of. “Similarly, as a pathologist distinguishes more extensive districts and then focuses on itemized cell structures, iStar can catch the overall tissue structures and furthermore center around the particulars in a tissue picture.”
To test the adequacy of the instrument, Li and her partners assessed iStar on a wide range of kinds of malignant growth tissue, including bosom, prostate, kidney, and colorectal diseases, blended in with solid tissues. Inside these tests, iStar had the option to naturally recognize growth and disease cells that were difficult to distinguish just by eye. Clinicians in the future might have the option to get and analyze all the more difficult-to-see or difficult-to-recognize malignant growths with iStar going about as a layer of help.
Notwithstanding the clinical potential outcomes introduced by the iStar procedure, the apparatus moves very immediately compared with other, comparable man-made intelligence instruments. For instance, when set up with the bosom malignant growth dataset the group utilized, iStar completed its examination in only nine minutes. On the other hand, the best contender, a simulated intelligence device, required over 32 hours to think of a comparable investigation.
That implies iStar was multiple times quicker.
“The ramifications are that iStar can be applied to countless examples, which is basic in huge-scope biomedical examinations,” Li said. “Its speed is additionally significant for its ongoing expansions in 3D and biobank test expectations. In the 3D setting, a tissue block might include hundreds to thousands of sequentially cut tissue cuts. The speed of iStar makes it conceivable to reproduce this gigantic measure of spatial information within a brief timeframe.”
Also, the equivalent goes for biobanks, which store thousands, if not millions, of tests. This is where Li and her associates are next, pointing out their examination and augmentation of iStar. They desire to assist scientists with acquiring better understandings of the microenvironments inside tissues, which could give more information to symptomatic and treatment inspirations pushing ahead.
More information: Daiwei Zhang et al, Inferring super-resolution tissue architecture by integrating spatial transcriptomics with histology, Nature Biotechnology (2024). DOI: 10.1038/s41587-023-02019-9