A copious stock of clean energy is on display. It is the hydrogen that we can get from water (H2O) using energy from renewable sources. In the fight against climate change, scientists are looking for low-cost ways to make clean hydrogen from water to replace fossil fuels.
Vehicles can be powered by hydrogen, which releases only water. Additionally, hydrogen plays a crucial role in numerous industrial processes, such as the production of ammonia and steel. In those sectors, using cleaner hydrogen is highly desirable.
A multi-institutional group driven by the U.S. Branch of Energy’s (DOE) Argonne Public Lab has fostered a minimally expensive impetus for an interaction that yields clean hydrogen from water. “A process called electrolysis produces hydrogen and oxygen from water and has been around for more than a century,” said Di-Jia Liu, senior chemist at Argonne. Other contributors include DOE’s Sandia National Laboratories, Lawrence Berkeley National Laboratory, and Giner Inc. Additionally, he holds a joint appointment at the University of Chicago’s Pritzker School of Molecular Engineering.
“We wanted to create a low-cost anode catalyst in a PEM electrolyzer that generates hydrogen at high throughput while using little energy. The cobalt-based catalyst developed by our technology could eliminate the main cost restriction in creating clean hydrogen in an electrolyzer.”
Di-Jia Liu, senior chemist at Argonne.
For this process, a new generation of technology is represented by PEM (proton exchange membrane) electrolyzers. At temperatures close to room temperature, they are able to split water more effectively into hydrogen and oxygen. They are an excellent choice for producing clean hydrogen from renewable but intermittent sources like wind and solar because they require less energy.
The catalysts for this electrolyzer’s cathode and anode electrodes are distinct. Hydrogen is produced by the anode catalyst, while oxygen is produced by the cathode catalyst. The use of iridium, which currently costs approximately $5,000 per ounce on the market, in the anode catalyst presents a challenge. The absence of supply and significant expense of iridium represent a significant boundary for the far-reaching reception of PEM electrolyzers.
The new catalyst’s primary component is cobalt, which is significantly less expensive than iridium. Liu stated, “We endeavored to develop a low-cost anode catalyst in a PEM electrolyzer that generates hydrogen at high throughput while using minimal energy.” By utilizing the cobalt-based impetus arranged by our technique, one could eliminate the fundamental bottleneck of cost in creating clean hydrogen in an electrolyzer.”
Utilizing its PEM electrolyzer test stations, Giner Inc., an electrolyzer and fuel cell research and development company, evaluated the new catalyst under industrial conditions. The presentation and strength far exceeded those of the contenders’ impetuses.
Understanding the atomic-scale reaction mechanism under electrolyzer conditions is crucial to improving the catalyst’s performance. Using X-ray analyses performed at the Advanced Photon Source (APS) in Argonne, the team was able to decipher the significant structural changes that take place in the catalyst when it is in use. Using electron microscopy at Sandia Labs and the Argonne Center for Nanoscale Materials (CNM), they also found important characteristics of the catalyst. Both the APS and the CNM are facilities used by the DOE Office of Science.
According to Argonne Materials Scientist Jianguo Wen, “We imaged the atomic structure on the surface of the new catalyst at various stages of preparation.”
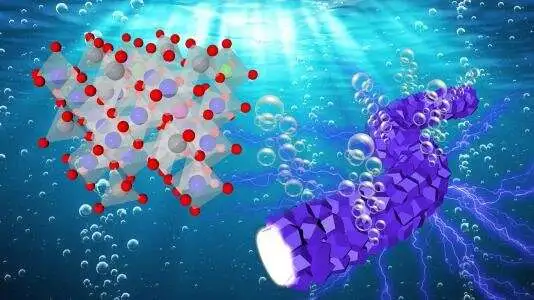
During the electrocatalytic reaction with water, oxygen bubbles develop from interconnected, fibrous catalyst particles (right). Left: Cobalt-based catalyst’s lattice structure. Credit: Argonne National Laboratory/Lina Chong and Longsheng Wu using a Shutterstock background
Likewise, computational displaying at Berkeley Lab uncovered significant bits of knowledge about the impetus’ strength under response conditions.
The group’s accomplishment is a step in the right direction in DOE’s Hydrogen Energy Earthshot drive, which emulates the U.S. space program’s “Moon Shot” of the 1960s. In a decade, it aims to bring down the cost of producing green hydrogen to one dollar per kilogram. At that price, green hydrogen production could change the country’s economy. Manufacturing, transportation, the electric grid, residential and commercial heating, and other uses include
Liu made the following remark: “More generally, our results establish a promising path forward in replacing catalysts made from expensive precious metals with elements that are much less expensive and more abundant.”
This examination was distributed on May 11 in Science.
More information: Lina Chong et al, La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis, Science (2023). DOI: 10.1126/science.ade1499





