To combat resistant bacteria, medical professionals need new antibiotics right away. Antimicrobial molecules that bind to novel targets in the metabolism of bacteria have been developed through the modification of the chemical structure of naturally occurring peptides by researchers at the University of Zurich and the company Spexis.
Bacteria that are resistant to the majority of commonly used antibiotics are responsible for the deaths of over five million people worldwide each year. To ensure that patients with bacterial infections can still be successfully treated, new antibiotics are required immediately. According to chemist Oliver Zerbe, who is in charge of the NMR facilities at the University of Zurich, “the development pipeline for new antibiotics is unfortunately fairly empty.” It’s been a long time since the last anti-toxins against beforehand unused objective particles were endorsed.”
In a recent concentrate distributed in Science Advances, Zerbe presently examines the improvement of a profoundly successful class of anti-toxins that battle gram-negative microbes in a clever manner. This group of bacteria is considered extremely dangerous by the WHO. Carbapenem-resistant enterobacteria are an example of this group, whose double cell membrane gives them a particularly strong resistance. The study also included researchers from the pharmaceutical company Spexis AG in addition to the UZH team.
“The new antibiotics were particularly successful at treating lung infections. In situations where most other antibiotics fail, they are also quite successful against enterobacteria that are resistant to carbapenem.”
Chemist Oliver Zerbe, head of the NMR facilities at the University of Zurich.
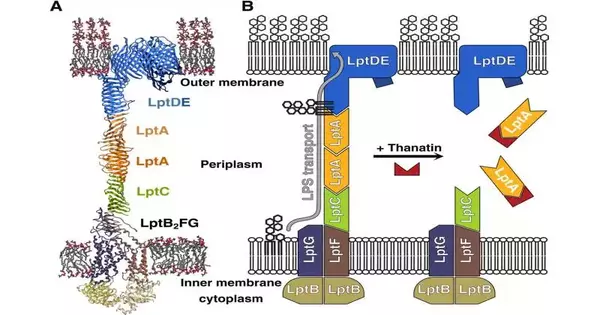
Chemically optimized natural peptide The study’s starting point was a naturally occurring peptide called thanatin that insects use to fight infections. A study conducted a few years ago by now-retired UZH professor John Robinson revealed that thanatin disrupts a crucial lipopolysaccharide transport bridge between the inner and outer membranes of gram-negative bacteria.
Accordingly, these metabolites develop inside the cells, and the microscopic organisms die. Thanatin, on the other hand, is not suitable for use as an antibiotic because, among other things, it is not very effective and bacteria quickly develop resistance to it.
The analysts in this manner altered the substance design of thanatin to upgrade the peptide’s attributes. ” “Strructural analyses were necessary for this,” says Zerbe. His group used nuclear magnetic resonance (NMR) to see where and how thanatin binds to and disrupts the bacterial transport bridge after synthesizing the various parts of the bridge.
Utilizing this data, analysts from Spexis AG arranged the substance changes that were important to support the peptide’s antibacterial impacts. Among other things, the molecule’s stability was improved by additional mutations.
The synthetic peptides were then tested in mice with bacterial infections, and the results were excellent. They were safe, effective, and resistant to resistance. “Especially for treating lung infections, the novel antibiotics proved to be very effective,” says Zerbe. In situations where the majority of other antibiotics fail, they are also highly effective against carbapenem-resistant enterobacteria.”
In addition, the newly developed peptides are stable in the blood for a longer period of time and are neither toxic nor harmful to the kidneys, and all of these properties are necessary for a drug to be approved. However, before the first tests on humans can begin, additional preclinical studies are required.
The researchers ensured that the most promising peptides for their study would also be effective against bacteria that have already developed thanatin resistance. According to Zerbe, “We are confident that this will significantly slow the development of antibacterial resistance.” A new class of antibiotics that is also effective against bacteria that are resistant may soon be available.
More information: Matthias Schuster et al, Peptidomimetic antibiotics disrupt the lipopolysaccharide transport bridge of drug-resistant Enterobacteriaceae, Science Advances (2023). DOI: 10.1126/sciadv.adg3683