A new composite material that outperforms individual compounds by one to two orders of magnitude was created by University of Twente researchers. Several earth-abundant elements make up the composite, which could potentially be used to produce hydrogen efficiently without platinum or other precious or rare metals. The findings were presented by the researchers in the journal ACS Nano.
The energy carrier of the future is thought to be green hydrogen. In essence, hydrogen provides a means of storing (green) energy for extended periods of time. Because of this, it is of the utmost significance to produce it as quickly as possible. One of the most environmentally friendly methods for producing green hydrogen is the electrolysis of water. However, either the process isn’t efficient enough or we need a lot of expensive and rare materials with the current electrolysis methods.
“At the moment, the most effective electrolyzers contain platinum and iridium, which are required for the electrodes that water uses to produce hydrogen and oxygen gas. Platinum, however, and iridium in particular, are too uncommon. That is the reason we’re continually searching for anode materials produced using more bountiful assets that can additionally be utilized as effective and stable electrocatalysts,” makes sense to UT-specialist Chris Baeumer. A compound with five distinct transition metals was exactly what the researcher and his team were looking for in a new material.
“Today, the most efficient electrolyzers comprise platinum and iridium, which are required for the electrodes that create hydrogen and oxygen gas from water. Nevertheless, platinum and, in particular, iridium are too scarce. That is why we are continuously looking for electrode materials made from more abundant resources that can also be employed as efficient and stable electrocatalysts.”
UT-researcher Chris Baeumer.
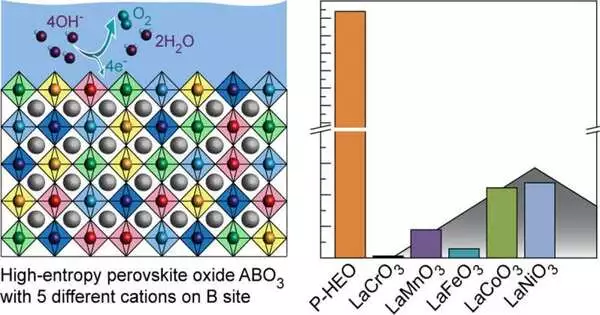
When used as a catalyst, each of the five transition metals is only moderately active. On the other hand, the researchers discovered that the total activity outperformed the individual compounds by as much as a factor of 680. Baeumer explains that the increased activity came as a surprise.
“When we started testing, it quickly turned out that the activity was also much higher. We expected that the stability would be better than that of traditional composites. We discovered, in collaboration with our collaborators from Karlsruhe (Germany) and Berkeley (U.S.), that transition metals can “help” each other make the whole material better than the sum of its parts in a so-called synergy effect.”
All electrodes cannot be directly replaced with this new material based on these new findings. Since combining the five distinct materials is difficult, the activity has only been tested in a laboratory setting thus far.
“We’re contrasting a newfound composite with materials upgraded for enormous scope creation, implying that our new material actually should be tried on the modern scale.” UT-postdoc Shu Ni, who is leading these future developments for materials optimization, explains that this combination of transition metals has the potential to outperform currently available alternatives with some tweaking and further research.”
More information: Mohana V. Kante et al, A High-Entropy Oxide as High-Activity Electrocatalyst for Water Oxidation, ACS Nano (2023). DOI: 10.1021/acsnano.2c08096