Whenever a non-metastatic cerebrum growth—a meningioma—repeats after a medical procedure and radiation therapy, a patient is out of choices. No medications are endorsed for these forceful cancers, which happen in up to 20% of cases and can prompt patient incapacity or even death.
In any case, presently, Northwestern Medicine researchers, in a global joint effort with researchers at the University of California, San Francisco and the University of Hong Kong, have distinguished a medication that represses the development of the most forceful meningiomas and how to most precisely recognize which meningiomas will respond to the medication.
The medication is a fresher disease treatment called abemaciclib.
The researchers showed the viability of the medication in select patients, mouse models, a 3D living tissue cerebrum growth (organoids), and cell societies.
“Our study identifies which patients we should treat with this drug, because their tumor will likely respond to it. Eventually we hope to tailor medical therapy to the genetic changes within each individual person’s meningioma,”
Dr. Stephen Magill, an assistant professor of neurological surgery at Northwestern University Feinberg School of Medicine
Agents found that meningiomas can be separated into sub-atomic subgroups with various clinical results and repeat rates. This new strategy for ordering cancers permits researchers to anticipate and repeat more precisely than the ebb and flow technique for grouping the growth.
Right now, after a medical procedure, specialists inspect an example of a cancer under a magnifying instrument and grade it one to a few in its forcefulness. Yet, the grade is just around 70% precise, meaning a few cancers will act in a manner that doesn’t fit with how they show up under the magnifying instrument.
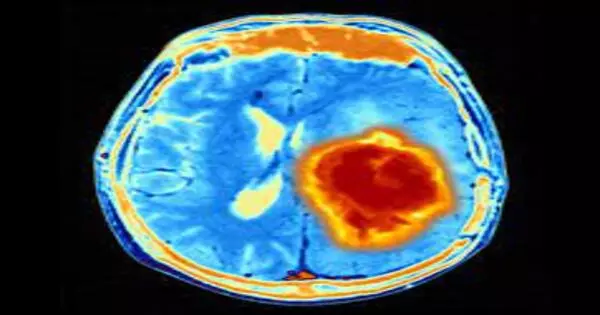
“Our review distinguishes which patients we ought to treat with this medication, in light of the fact that their cancer will probably respond to it,” said concentrate on pioneer and relating creator, Dr. Stephen Magill, an associate teacher of neurological medical procedure at Northwestern University Feinberg School of Medicine and a Northwestern Medicine doctor. “We can currently give them choices and expectations for a more drawn-out, side-effect free life.”
Magill is also a member of Northwestern University’s Robert H. Lurie Comprehensive Cancer Center.
The paper was published today in Nature Genetics.
Meningiomas are the most well-known essential (non-metastatic) growth in the focal sensory system, with around 31,000 individuals determined to have a meningioma consistently in the U.S. The side effects are migraines, seizures, or neurological deficiencies (shortcomings, vision misfortune, twofold vision, or tangible changes).
The medication is a cell cycle inhibitor, meaning it impedes the cell division cycle and represses cancer development.
“Ultimately, we desire to fit clinical treatment to the hereditary changes inside every distinctive individual’s meningioma,” Magill said.
Agents concentrated on atomic changes in the cancer to grasp what drives development and plan treatments to focus on the Achilles impact point of the growth.
“We can track down a shortcoming in that cancer, put a stick in the spokes and prevent it from developing,” Magill said.
The new review was led by DNA methylation profiling and RNA sequencing of 565 meningiomas. This empowered specialists to see what qualities are communicated by the cancer and the degree of articulation, uncovering a mark of the DNA.
“By doing that, we observed three separate gatherings of meningiomas based on their science,” Magill said. “For each gathering, we observed an alternate organic component advancing the cancer’s development, with each gathering having an alternate clinical result.”
These gatherings are not quite the same as the past reviewing framework and “are more precise at foreseeing the clinical way of behaving of the growth,” Magill said.
Researchers found that forceful cancers have numerous atomic changes in a typical pathway of cell division that empower the cells to isolate more and return after a medical procedure.
“We contemplated whether by repressing that pathway we could prevent the cancers from developing,” Magill said. “We tried that in more than one way and observed it was valid in patients, mouse models and cell societies.”
Mice with meningiomas treated with the prescription lived longer, and their tumors didn’t develop as quickly. The medication was likewise utilized off-label as merciful use in a few patients whose cancers diminished in size and whose side effects improved, suggesting the medication ought to be considered for clinical preliminaries, Magill said.
The following stages in the exploration are to approve these discoveries in extra populations and expand on them to decide if we can utilize sub-atomic elements to foresee which meningioma patients ought to be treated with radiation notwithstanding medical procedure.
Researchers intend to decipher these discoveries and techniques to make this sub-atomic profiling generalizable and accessible to all patients with meningioma.
Researchers approved their discoveries in a free accomplice by teaming up with specialists at the University of Hong Kong.