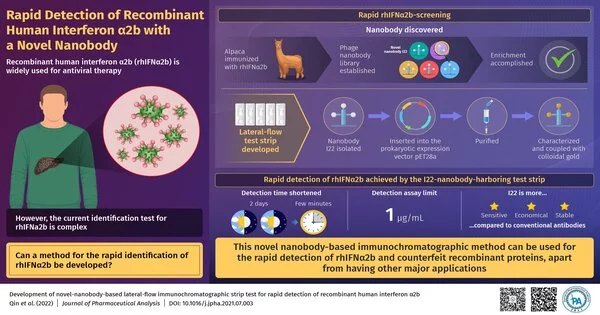
Interferons are proteins that play an important role in our normal defense mechanisms.These proteins also display a noteworthy antiviral action. The recombinant human interferon 2b (rhIFN2b) was endorsed by the U.S. Food and Drug Administration in 1986. From that point onward, it has been utilized as an antiviral specialist for the treatment of hepatitis B and hepatitis C. In spite of its far-reaching applications, nonetheless, there remains an issue: the recognition of rhIFN2b is monotonous and tedious.
In another review, scientists from China recently fostered a clever technique for the quick and proficient recognition of rhIFN2b. This paper was made accessible via the web on July 8, 2021, and was distributed in the Journal of Pharmaceutical Analysis on April 30, 2022.
To foster their new technique, they immobilized a clever nanobody on a paper strip. The nanobody utilized in this strategy was initially gotten from an alpaca — a type of South American camelid vertebrate. In this manner, it was produced in the exploration research facility utilizing recombinant DNA innovation—a method used to subclone DNA sections to get high amounts of manufactured proteins. This is typically accomplished by utilizing microbes or other prokaryotic cells. A nanobody is a utilitarian section of a bigger immune response. As the immobilized novel nanobody ties rhIFN2b firmly and with high specificity, it was utilized for the quick and secure recognition of rhIFN2b.
“The lateral flow immunochromatography assay using nanobodies has a great potential to replace standard antibody-based ligand-binding assays for a rapid identification test of recombinant protein therapies due to the advantages of nanobodies in reagent preservation, manufacture, and cost.”
Dr. Junzhi Wang
As indicated by Dr. Junzhi Wang, “Attributable to the benefits of nanobodies in reagent safeguarding, creation, and cost, the parallel stream immunochromatography measure utilizing nanobodies has a high potential to supplant conventional neutralizer-based ligand-restriction examines for a quick and distinguishing proof trial of recombinant protein therapeutics.”
The examination group described the limiting conditions for the I22-rhIFN2b cooperation, for example, restricting between nanobody 122 and rhIFN2b, utilizing an Octet stage. The obtained information plainly demonstrated a tight restriction. The limiting particularity was additionally approved utilizing Western blotting, a procedure used to recognize proteins utilizing protein-explicit antibodies.
“The rhIFN2b items right now accessible in China incorporate infusions, infusion powders, gels, and glues. The immunochromatography strips must be utilized to assess fluids or items in powder form that can be broken up and applied to the strips. This is on the grounds that the item needs to diffuse along the strip by means of fine activity; gels and glues don’t fulfill this necessity, “which makes sense of Dr. Wang.”
Strangely, the created rhIFNa2b location has an identification breaking point of 1 g/mL, which is lower than as far as possible. This makes it a more touchy lab-based procedure for fast ID of rhIFN2b. Another large benefit is the utilization of nanobodies for protein identification. This is on the grounds that nanobodies can be gotten in a conservative way by collecting modest bacterial cells. Also, huge volumes of nanobodies can be obtained with no sweat by utilizing regularly utilized lab methods.
(Dr. According to Wang, “The activity season of rhIFN2b ID was abbreviated from two days to a couple of moments with our test.” It can, thusly, address the issues of quick location of this group of recombinant protein items and give a solid foundation to work on the proficiency of market fake recognition. Later on, fast identification could be completed in an overall way. “
In summary, the recently evolved technique could allow for the smoother, quicker, and precise discovery of recombinant or misleadingly produced proteins, allowing for early analysis and treatment of hepatitis.
More information: Xi Qin et al, Development of novel-nanobody-based lateral-flow immunochromatographic strip test for rapid detection of recombinant human interferon α2b, Journal of Pharmaceutical Analysis (2021). DOI: 10.1016/j.jpha.2021.07.003