Lately, the buzzword in valuable metals is palladium.
The demand for this intriguing, brilliant white change metal continues to outstrip supply, pushing its price per ounce far above gold and silver.
Palladium and other uncommon, exorbitant valuable metals, for example, platinum, iridium, and ruthenium, are also important in substance changes, particularly metal catalysis, which has turned into a fundamental instrument for assembling complex atoms in the advancement of pharmaceuticals, polymers, and other useful synthetic compounds.
Because of the scarcity and high cost of these valuable metals, there is a need to foster impetus from progress metals that are more plentiful and, for the most part, less expensive, such as nickel, a cousin of palladium.
Thus, the last ten years have seen an emotional extension of new synergist bond-framing changes, including nickel.
“We know from the writing that nickel edifices are incredibly helpful in playing out certain changes, perhaps better than other progress metals out there,” said Liviu Mirica, William H. and Janet G. Lycan Professor of Chemistry at the University of Illinois at Urbana-Champaign. “Individuals have improved at streamlining conditions for explicit changes, so we are gradually getting to where nickel could match palladium in these changes.”
“From the literature, we know that nickel complexes are quite useful in conducting some transformations, possibly better than other transition metals out there. People have gotten extremely adept at optimizing circumstances for specific transformations, so we are gradually approaching the point where nickel may compete with palladium in these transformations.”
said Liviu Mirica, William H. and Janet G. Lycan Professor of Chemistry at the University of Illinois at Urbana-Champaign.
All the more as of late, researchers have been zeroing in on creating nickel impetuses that can be straightforwardly photoinitiated by light, which Mirica said has been demonstrated to be an extremely effective area of examination. It is already conceivable to deliver responses to that poor person.
They actually require the utilization of an extra photocatalyst—commonly based on valuable metals, for example, iridium or ruthenium, which are much more costly than palladium.
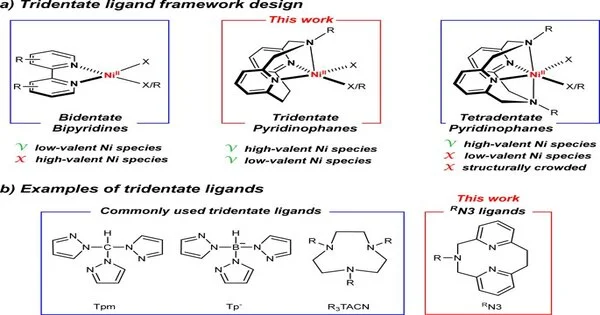
In a paper as of late distributed in Nature Communications, Mirica and postdoctoral analyst Hanah Na report their work on the advancement of a totally novel tridentate ligand that is directed with nickel to make an impetus that can be straightforwardly enacted by light to frame a carbon-oxygen security without the utilization of an extra photocatalyst. C-O bonds are pervasive in numerous regular items, drugs, and agrochemicals.
Mirica and Na trust their new class of tridentate pyridinophane ligands (RN3) can prompt the improvement of new nickel impetuses and is a pragmatic stage for definite mechanical investigations of other nickel-catalyzed compound responses.
“It is an equipped impetus, and on top of that, it can really do this photocatalysis without help from anyone else; it doesn’t need these other photocatalysts,” Mirica said. “It opens up numerous roads of exploration that we think could be utilized for some extra applications.”
These tridentate pyridinophane ligands (RN3) expand on past work by Mirica, who had previously fostered a clever four-pronged atom known as a tetradentate ligand, whose design looks like the pocket of a mitt. This ligand structure advanced fast C bond shaping reactivity while additionally settling the higher oxidation conditions of nickel.
It’s entirely steady. However, those intermediates over the course of the last ten years have been excessively steady. They’re not skilled in reactant applications, “Mirica said.
Then there is the bidentate ligand structure bipyridyl that most scientific experts are utilizing in nickel photocatalytic processes, which gives upgraded reactivity and the capacity to change advancement to get the ideal response.
“It’s incredible for reactant science, yet you can’t disengage or see these exceptional nickel species,” Mirica said.
Ordinarily, Mirica made sense of, exemplary natural scientific experts have a specific compound change at the top of their priority list and attempt anything that impetuses them to think will be great, and anything conditions or added substances would be valuable and streamline it, zeroing in on an unmistakable change.
“We have a somewhat unique methodology: a metallo-driven approach, and for this situation, nickel is the metal of interest,” he said. “I’m keen on having the option to configure, disconnect, and portray nickel edifices with various coordination numbers, different ligand conditions, and in various oxidation states, which at last will direct their reactivity.”
This most recent ligand structure is somewhere close to the next two.
“We open up a coordination site, we open up that nickel place, by eliminating one of the four nitrogens, to permit different things to tie to it, and ultimately, it permits you to do synergist movement, yet at the same time have the option to detach and portray intermediates,” he said.
Their novel tridentate ligand empowered them to uncover the key response steps and halfway species in this reactant cycle. The report says that an inside-and-out unthinking comprehension of Ni-interceded photocatalysis is fundamental for objective response planning and advancement of the nickel-intervened compound cycle, which the scientists make sense of.
Their unthinking review utilized strategies including atomic attractive reverberation (NMR), electron paramagnetic reverberation (EPR), in situ infrared (IR) spectroscopy, electrochemical and photophysical estimations, and computational investigations.
From a mechanical viewpoint, the photocatalytic cycle is surely known, but the Ni-interceded redox cycle has stayed a secret. Paramagnetic Ni (I) and Ni (III) species are thought to be important for the cycle, yet have not been totally researched, and the vital reactant steps of oxidative expansion, trans-metalation, and reductive disposal at the nickel places have never been straightforwardly noticed.
For a very long time, Na made sense of. Noticeably, light-intervened photoredox catalysis has made imperative commitments in the field of engineered natural science. Growing new systems and response condition improvement are frequently accomplished by experimentation instead of being founded on an exhaustive understanding of the hidden response instrument.
Na said this may be on the grounds that comprehension of basic science requires a significant commitment from the inorganic and organometallic science fields (past the extent of the exploration interests in engineered natural science), including the amalgamation and portrayal of related metal edifices and investigation of their photochemistry and photophysics.
“As inorganic and organometallic scientific experts, we need to add to this arising research field, generally zeroing in on unwinding signs to figure out fundamental response components — which isn’t highly finished by natural physicists,” Na said. “We accept that our work would give critical knowledge into the response plan and quest for new substance changes in the prospering field of photoredox catalysis, and along these lines can affect both the natural and inorganic science local area.”
The objective, Mirica made sense of, is to release new reactivity that could eventually be useful to natural scientists, who could then utilize this framework and use it for extremely specific engineered targets.
“They may not work now as well as the finely advanced or finely tuned frameworks that individuals use consistently in a natural lab, but we trust that our new Ni impetuses will be regularly utilized quite a while down the line,” Mirica said.