Cheap iron salts are a vital aspect of working on the production of fundamental forerunners for drugs and different synthetics, as per researchers at Rice College.
They’ve refined the most common way of creating diazides and building block atoms in the development of medications and rural synthetics. Iron salts, along with processes called extremist ligand move and ligand-to-metal charge move (LMCT), make it reasonable and harmless to the ecosystem.
Rice-engineered physicist Julian West and co-lead creators Kang-Jie (Harry) Bian and Shih-Chieh Kao, both alumni understudies in his lab, report in Nature Interchanges that illuminating their reagents with apparent light permits them to shape diazides in conditions that are definitely more delicate than current modern cycles that normally include high intensity and destructive acids.
Diazides are atoms with two amine bunches that can be functionalized, meaning they can undoubtedly respond with different particles. Contingent upon how they’re built, they can be the basis of numerous helpful mixtures.
West and his colleagues used extreme ligand move (or “revolutionary bounce back”) in a new report to add two useful gatherings to a single alkene, natural particles derived from petrochemicals that contain at least one carbon two-fold bond.
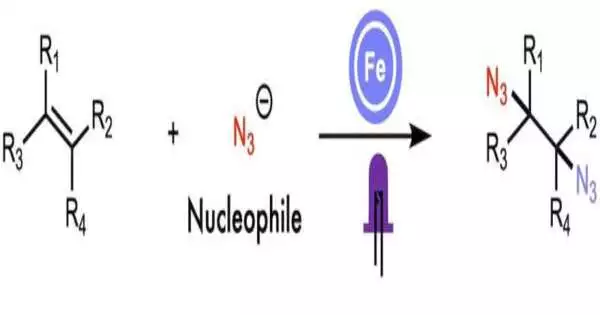
The synergistic collaboration of ligand-to-metal charge move and extremist ligand move produces diamines, building block atoms used in the creation of medications and rural synthetics. Rice College scientists presented their light-determined process in Nature Correspondences.
The method, along with the iron-interceded ligand-to-metal charge move, proved to be useful as they fabricated comparable antecedents called vicinal diazides out of normal feedstocks.
“It just consists of two reagents, iron nitrate and TMS azide, which each engineered lab will have,” said West, an associate teacher of science whose lab endeavors to work on drug production. “Essentially, you combine them as one in a typical dissolvable and focus light on it.” Practically every drug lab will have Driven lights. So essentially, they’ll simply pull things off the rack.
West said the extremist ligand move was roused by science, “remembering the proteins for our own livers.” There are proteins in nature that move iotas or parts of particles to an extremist to create another bond that can assist with developing larger atoms. We were eager to investigate the capability of that one stage in the last review.
“In this task, now that we’ve laid out how that functions, we can begin to join it with new moves toward making something else,” he said. “As with everything in natural science, nature recognized quite some time ago that this can be truly beneficial.”
Both LMCT and extremist ligand movement occur consistently when the reagents and arrangement are enlightened in the surrounding conditions. The lab figured out how to boost the cycle through stream science by running the arrangement through a circling cylinder and simply lighting that cylinder.

This enlightened circle rig assists Rice College physicists with utilizing stream photochemistry to create diamines, building block atoms in the development of medications, and rural synthetics.
“The response occurs in the part where you focus the light,” West said. “That way, we can handle more than a single clump and have a lot more control over how much light it gets by accelerating or slowing down the stream.”
“It’s simple to dump the salts in the jar and focus a light on it; yet to make a ton or improve it, the stream functions admirably,” he said.
“We figure it will be useful for labs that believe a simple way should be taken to make this sort of item, particularly in the event that they lack the opportunity to tweak and battle with getting these different strategies to function admirably,” West said.
Concentrate on the co-creators and incorporate Rice students David Nemoto Jr. and Xiaowei Chen.
More information: Kang-Jie Bian et al, Photochemical diazidation of alkenes enabled by ligand-to-metal charge transfer and radical ligand transfer, Nature Communications (2022). DOI: 10.1038/s41467-022-35560-3
Journal information: Nature Communications





