The outcome of COVID-19 immunizations is an incredible illustration of quality medication’s huge potential to forestall viral diseases. One reason for the antibodies’ prosperity is their utilization of lipid nanoparticles, or LNPs, to convey fragile messenger RNA to cells to create and help resistance. LNPs (small fat particles) have grown in popularity as a transporter for various quality-based medications to cells, but their application is complicated because each LNP must be specifically tailored to the beneficial payload it transports.
A group led by Hai-Quan Mao, a Johns Hopkins materials researcher, has made a stage that shows a vow to accelerate the LNP configuration cycle and make it more reasonable. The new methodology, likewise, can be adjusted to other quality treatments.
“Essentially, what we have done is develop a strategy that screens lipid nanoparticle parts and their extents to rapidly help recognize and make the ideal plan for use with various remedial qualities,” said Mao, director of the Institute for NanoBioTechnology at the Johns Hopkins Whiting School of Engineering and a professor in the Materials Science and Engineering and Biomedical Engineering branches.
The group’s review was distributed as of late in Nature Communications.
A vital element of powerful medicines is the way quality medication endures once it arrives at the objective cell. Sadly, the power of mRNA starts to decline in the span of 24 hours following its conveyance by LNPs. A promising option is plasmid DNA—a sturdier, twofold abandoned round DNA that can keep going for as long as seven days and hence can possibly further develop the treatment results of metabolic illnesses and diseases influencing the liver, where the helpful term is basic.
“This platform is versatile in that it is not restricted to pDNA delivery but may be easily expanded to the production of LNPs for a wide range of therapeutic gene payloads, as well as alternate delivery routes such as oral, intramuscular injection, or inhalation approach,”
Yining Zhu, an INBT researcher and biomedical engineering Ph.D
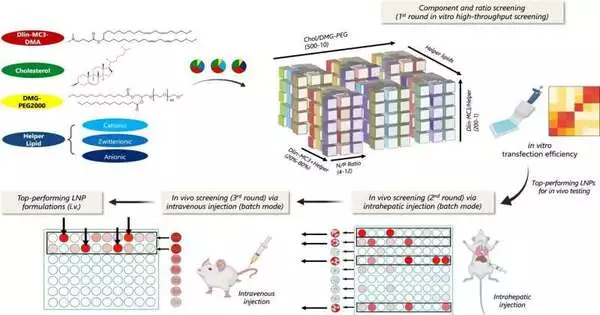
The lead creator, Yining Zhu, an INBT scientist and biomedical design Ph.D. understudy, as well as researchers from Johns Hopkins and the University of Washington, recognized the best LNP plan for pDNA conveyance to liver cells in this work. Their foundation screens LNPs bit by bit, tending to the physiological boundaries a LNP experiences as it explores the body to arrive at its objective. The stage assisted the group in recognizing the best LNPs from a library of in excess of 1,000 mixes.
“This stage is flexible in that it isn’t only restricted to pDNA conveyance, yet can be handily extended to the improvement of LNPs for an extensive variety of helpful quality payloads, as well as elective conveyance courses like oral, intramuscular infusion, or inward breath strategy,” said Zhu.
The scientists are currently working with Sean Murphy, an academic partner at the University of Washington, and his group to develop a jungle fever immunization that targets the disease-causing parasite during its lifecycle in the liver. This screening stage shows incredible guarantee to help speed up other LNP item advancements to further push the limits of quality medication, antibody improvement, and other novel therapeutics.
More information: Yining Zhu et al, Multi-step screening of DNA/lipid nanoparticles and co-delivery with siRNA to enhance and prolong gene expression, Nature Communications (2022). DOI: 10.1038/s41467-022-31993-y
Journal information: Nature Communications