The primary obstacles facing energy research are climate change and the scarcity of fossil fuels. One of the most promising options for addressing both of these issues is the clean fuel hydrogen that is produced by polymer electrolyte fuel cells (PEFCs).
However, the large amount of platinum (Pt) that PEFCs require makes their production and operation costly. Besides, how much Pt is in the world’s outside, and that means that to make PEFCs really reasonable, it is basic to diminish how much Pt that they use.
By and by, PEFCs use cathodes (the positive anode) made with Pt nanoparticles (NPs) that are supported on carbon black (Pt NPs/CB). However, recent studies have shown that Pt nanoclusters (Pt NCs) perform better than Pt NPs due to their higher oxygen reduction reaction (ORR) activity. Hitherto, the justification for Pt. NC’s high ORR movement has been muddled.
“We concentrated on Pt NCs derived from a Pt, carbon carboxylate (CO), and triphenylphosphine (PPh3) base, i.e., [Pt17(CO)12(PPh3)8]z (where z = 1+ or 2+).” We recently demonstrated that, unlike other Pt NCs, these are stable in air. We then used density functional theory (DFT) calculations to figure out what was causing its unusual activity.”
Professor Yuichi Negishi from Tokyo University of Science (TUS).
A new Pt NC with an ORR activity that is 2.1 times higher than that of commercial Pt NPs was recently developed by a team led by Professor Yuichi Negishi from the Tokyo University of Science (TUS). They also found out how this high activity came about.
“The Pt NCs derived from a Pt, carbon carboxylate (CO), and triphenylphosphine (PPh3) base, [Pt17(CO)12(PPh3)8]z (where z = 1+ or 2+), were the primary focus of our investigation. In contrast to other PTNs, we recently demonstrated that these are air-stable. “The reason for its remarkable activity was then revealed by density functional theory (DFT) calculations,” says Prof. Negishi.
The exploration group likewise included junior academic administrator Tokuhisa Kawawaki from the Tokyo College of Science, academic administrator Kenji Iida from Hokkaido College, teacher Toshihiko Yokoyama from the Organization for Sub-atomic Science, Japan, and teacher Gregory F. Metha from the University of Adelaide, Australia. On March 24, 2023, the study was published in the journal Nanoscale.
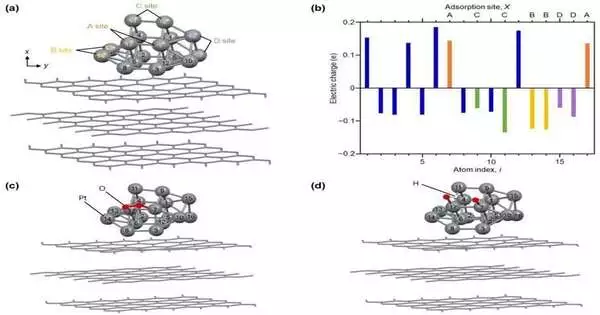
The Pt NCs were prepared by a calcination reaction and the adsorption of [Pt17(CO)12(PPh3)8]z onto carbon black. Using a method known as linear sweep voltammetry, they then compared its performance to that of conventional Pt NPs and CBs. They found that the original Pt NCs had better execution than the Pt NPs or CBs. Notably, the activity of the Pt NCs was 2.1 times higher than that of the Pt NPs and CB at 0.9 volts. Additionally, they discovered that the PT NCs were more durable than the commercial PT NPs and CBs and that an increase in Pt loading in the electrode results in an increase in its mass activity.
Then, they used DFT calculations to figure out where the high activity came from. Prof. Negishi says, “Our calculations suggest that the surface Pt atoms, which have an electronic structure suitable for the advancement of ORR, are the cause of the high ORR activity of the novel Pt NCs.”
Future high-activity, high-performance Pt catalysts for use in PEFCs can be designed using these findings as a model, advancing our efforts to combat climate change and the fossil fuel crisis.
More information: Tokuhisa Kawawaki et al, Pt17 nanocluster electrocatalysts: preparation and origin of high oxygen reduction reaction activity, Nanoscale (2023). DOI: 10.1039/D3NR01152F