The potential for superatomic material synthesis from superatomic molecules containing noble metal elements like gold and silver is the subject of research. In any case, the comprehension of silver-based superatomic atoms has been restricted. To fill this void, Japanese researchers investigated two silver-containing bimetallic superatomic molecules to ascertain the key factors that permitted their formation. In the future, it is anticipated that their findings will help advance the creation of novel materials.
As superatoms for the synthesis of materials with unique properties and potential new applications, noble metal nanoclusters like gold (Au) and silver (Ag) have gained attention in recent decades. These superatoms—also known as “artificial atoms”—have distinct properties from their bulk counterparts and typically consist of a cluster of a few to several hundred atoms. However, the formation of a closed-shell electron structure determines these superatoms’ stability, much like that of real atoms.
“So far, we humans have created a wide range of useful materials from the elements at our disposal on Earth.” However, with complicated energy and environmental challenges in the future, the creation of materials with novel qualities and uses is sought.”
Prof. Negishi
In comparison to Au-based superatoms, Ag-based superatoms are known for their superior properties and functions, such as selective catalytic activity and photoluminescence. However, the majority of research in this area has primarily focused on superatomic molecules with an Au base.
To fill this knowledge gap, Japanese researchers investigated the processes that lead to the formation of Ag-based superatomic molecules. On March 28, 2023, this study was published in the journal Communications Chemistry.
Discussing the inspiration behind concentrating on Ag-based superatoms, Prof. According to Negishi says, “Up until this point, we people have made different valuable materials from the components accessible to us on the planet. However, considering the complex energy and environmental issues of the future, it is desirable to develop materials with novel properties and functions.”
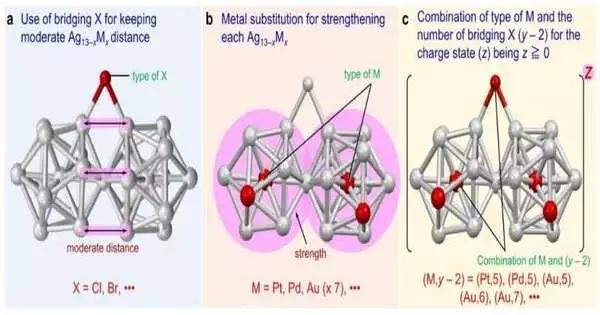
To this end, the analysts orchestrated two di-superatomic atoms with bromine (Br) as the crossing over ligand: [Ag23Pt2(PPh3)10Br7]0 and [Ag23Pd2(PPh3)10Br7]0, where PPh3 is triphenylphosphine. The first was made up of two Ag12Pt superatoms that were icosahedral and shared a vertex. Platinum atoms (Pt) were in the center of each superatom. The other superatomic molecule, on the other hand, had palladium (Pd) as its central atom and consisted of two icosahedral Ag12Pd structures.
The stability and geometric/electronic structure of these two nanoclusters were then compared to [Ag23Pt2(PPh3)10Cl7]0 (1) and [Ag23Pd2(PPh3)10Cl7]0 (2), two nanoclusters with chlorine (Cl) serving as the bridging atom and exhibiting geometrical similarity to the synthesized nanoclusters.
The researchers discovered a twist between the two icosahedral structures containing Br as the bridging ligand when they examined the four nanoclusters’ geometric structures. The researchers hypothesize that by reducing the distance between the two icosahedral structures, this twist stabilizes the nanocluster.
The PPh3 molecule moved further away from the metal nanocluster’s long axis, and the bond lengths of the Ag-P and Ag-Ag bonds changed as a result of the larger Br atom’s introduction of steric hindrance. According to these findings, the formation of metal nanoclusters is not hindered by the type of bridging halogen, despite the fact that it has a slight impact on their geometric structures.
Prof. Negishi explains, “As long as the bridging halogen is large enough to maintain a moderate distance between the two Ag12M structures, it appears to have little effect on whether superatomic molecules can be formed or not.”
However, the number of bridging halogens attached to the nanocluster was largely responsible for its stability. Stable metallic nanoclusters require a full valence shell, just like atoms do. The researchers were only able to attach a maximum of five bridging halogens to the prepared nanoclusters, which had a total of 16 valence electrons, in order to keep the metal nanocluster in a stable neutral or cationic state.
The presence of Pd and Pt focal molecules was viewed as the result of the development of metallic nanoclusters. Subbing the focal particle of Ag13 with Pt or Pd prompted an expansion of the normal restricting energy inside the nanoclusters, making it ideal for the arrangement of superatomic atoms.
In total, the researchers discovered three crucial requirements for the formation and isolation of two Ag13xMx structures connected by vertex sharing in superatomic molecules. These incorporate the presence of a spanning halogen that can keep an ideal separation between the two designs, a blend of heteroatoms and connecting incandescent light that outcomes in 16 valence electrons, and the development of an icosahedral center that is more grounded than Ag13.
“These findings offer clear design guidelines for the creation of molecular devices with various properties and functions and can potentially contribute to resolving pressing concerns regarding clean energy and the environment,” Prof. Negishi wrote in his paper.
More information: Sayuri Miyajima et al, Key factors for connecting silver-based icosahedral superatoms by vertex sharing, Communications Chemistry (2023). DOI: 10.1038/s42004-023-00854-0