In view of messenger RNA, or mRNA, might possibly treat many ailments, including malignant growth, hereditary illnesses, and, as the world has learned lately, lethal infections.
To work, these medications should be conveyed straightforwardly to target cells in nanoscale air pockets of fat called lipid nanoparticles, or LNPs—mRNA isn’t a lot of good if it doesn’t arrive at the right cell type.
A group of scientists at the Georgia Institute of Technology and Emory University’s School of Medicine has moved toward further developing improvement of these hand-crafted conveyance vehicles, revealing their work June 30 in Nature Nanotechnology. Curtis Dobrowolski and Kalina Paunovska, students in the lab of James Dahlman, have fostered a framework to make pre-clinical nanoparticle concentrates more prescient. Their revelations, as of now, are impacting the course of exploration in this developing, serious field.
“I’m extremely amped up for this review and expect to move the majority of our future tasks to this system,” said Dahlman, academic partner and McCamish Foundation Early Career Professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory.
Sequencing of Occasions
For the past couple of years, Dahlman has cooperated with Coulter BME Professor Philip Santangelo in a bustling examination venture. Santangelo’s lab creates mRNA treatments, and Dahlman’s lab conveys them utilizing LNPs.
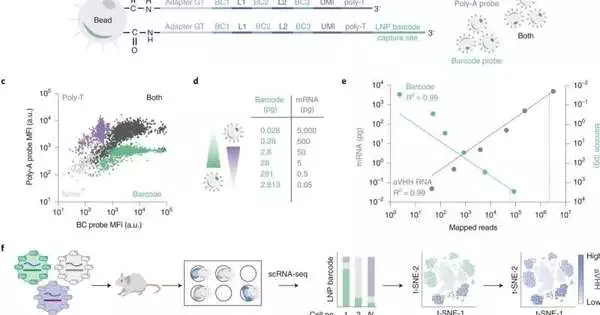
To accelerate the most common way of testing the viability of their LNPs, Dahlman’s group has fostered a method called DNA barcoding. In this cycle, scientists embed a bit of DNA that relates to a given LNP. The LNPs are then infused and the cells are analyzed for the presence of the “scanner tags” utilizing hereditary sequencing. The framework recognizes which scanner tags have arrived at which explicit targets, featuring the most encouraging nanoparticles. Since numerous DNA groupings can be perused immediately, the barcoding system permits many tests to be performed all the while, thus speeding up the revelation of viable lipid nanoparticle transporters.
DNA barcoding has altogether worked on the nanoparticle pre-clinical screening process. Yet, there is as of yet a huge boundary influencing drug conveyance. Due to their variety, cells are similar to moving targets. Dahlman noticed that cells recently thought to be homogeneous are made up of particular and changed cell subsets. His group determined that this compound and hereditary heterogeneity affect how well LNPs can convey mRNA treatments into the cells.
“Cells don’t have just one protein that characterizes them—they’re muddled,” Dahlman said. “They can be characterized by a mix of things, and truth be told, they are best characterized by utilizing every one of the qualities they do, or don’t, express.”
To test their speculation, the scientists created another device to gauge these things immediately. Their multiomic nanoparticle conveyance framework is called single-cell nanoparticle focusing on sequencing, or SENT-seq.
Multiomics approach
Utilizing SENT-seq, the analysts had the option to measure how LNPs convey DNA scanner tags and mRNA into cells, the ensuing protein creation worked with by the mRNA drug, as well as the character of the cell, in a great many individual cells.
This multiomics approach could provide a significant jump forward for high-throughput LNP revelation. The SENT-seq method permitted the group to recognize cell subtypes that show especially high or low nanoparticle take-up and the qualities related to those subtypes.
Thus, as well as testing the viability of a medication and how certain cell subtypes respond to nanoparticles, they’re recognizing which qualities are engaged with the fruitful take-up of LNPs. They’re doing everything simultaneously.
“The information proposes that these different cell subsets have particular reactions to nanoparticles that impact how well a mRNA treatment functions,” Dahlman said. “There’s still a ton of work to be done, yet we figure the capacity to all the while read out high-throughput nanoparticle conveyance and the cell’s reaction to nanoparticles will prompt better mRNA treatments.”
Co-lead creator Paunovska said that she and Dobrowolski thought of the idea for the SENT-seq framework “naturally, following two months of cooperating.”
Dahlman added: “I’m grateful for the work that Curtis, Kalina, and the group did in the lab.” I think this is the start of a very fascinating stage with regards to our work. “
More information: Curtis Dobrowolski et al, Nanoparticle single-cell multiomic readouts reveal that cell heterogeneity influences lipid nanoparticle-mediated messenger RNA delivery, Nature Nanotechnology (2022). DOI: 10.1038/s41565-022-01146-9