All cell films in the human body have implanted proteins that act as sensors, couriers, or for moving and managing substances going all through the cell. Transport proteins specifically are inadequately perceived due to their primary intricacy and their hydrophobic nature that makes them impervious to study. Simultaneously, these vehicle proteins, particularly those that direct glucose, play a crucial part in the development of harmful cancers.
In another review, researchers led by Dr. Shuguang Zhang, Ph.D. of the MIT Media Lab, show a strategy for rapidly foreseeing the plans of hydrophilic variation designs of the 14 glucose transport film proteins in cells. This will permit analysts to more effectively concentrate on the proteins in water. The researchers confirm the accuracy of the predicted designs by comparing them to previous crystallographic images of two of the proteins.
They trust that an upgraded understanding of these glucose transport proteins will speed the advancement of helpful monoclonal antibodies to treat disease metastasis. This would basically starve disease cells by impeding glucose carriers.
“To meet their insatiable energy need, most cancer cells greatly boost their expression and production of glucose transporters known as GLUTs. Because GLUTs have a difficult structure, there are basically no effective medications to block them right now.”
Eva Smorodina
“Most disease cells essentially increase their demeanor and the creation of glucose carriers, called GLUTs, for their voracious energy interest,” says Eva Smorodina, an undergrad understudy in primary science at the Greiff Lab, University of Oslo, who is the first creator of a paper on the review distributed June 27 in QRB Discovery. “Presently, there are basically no viable medications to hinder GLUTs, since they have a difficult design.”
The GLUTs’ perplexing designs contain 12 transmembrane hydrophobic helices implanted in the film. In their local or glasslike express, the hydrophobic designs should be put in a unique cleanser or reagent for lab study, or they lose their construction. Also, with proteins, design and capability are permanently related.
“Concentrating on these proteins in cleanser resembles wearing weighty gloves to gather a costly watch or play a violin,” says Dr. Zhang, one of a handful of biomedical researchers for whom the investigation of film proteins is crucial as it could be crucial to how we might interpret disease cells. He initially started dealing with film proteins in the mid 2000s. “Not many people were focusing on these film proteins,” Dr. Zhang says.Because of their inborn protection from study, “they’re like a hot potato,” he says, referring to their inborn protection from study.
The new work depends on Dr. Zhang’s prosperity a long time back, when he and a group achieved what Dr. Zhang had been dealing with for almost 10 years: they planned a perfectly basic strategy called the QTY code for changing a hydrophobic cell film protein into a hydrophilic protein by subbing numerous hydrophobic amino acids.
The QTY code is named for the images of the three amino acids — glutamine (Q), threonine (T) and tyrosine (Y) — that are filled in for four hydrophobic amino acids: leucine (L), isoleucine (I), valine (V) and phenylalanine (F). None of these amino acids carries a charge, which makes the replacement harmless. The structure is vital for the working of the proteins, and the replacement doesn’t adjust the design.
In the most recent review, Dr. Zhang and her group apply the QTY code to the 14 glucose transport film proteins that transport sugar to cells. They utilized the new AlphaFold2 program, a man-made reasoning-based computational program created by the organization DeepMind, that can precisely and immediately foresee how proteins crease. Dr. Zhang and her group utilized the QTY code with the open-source AlphaFold2 to foresee the alpha helical states of the 14 GLUT proteins in both their normal hydrophobic shapes and their QTY-code changed water-solvent shapes.
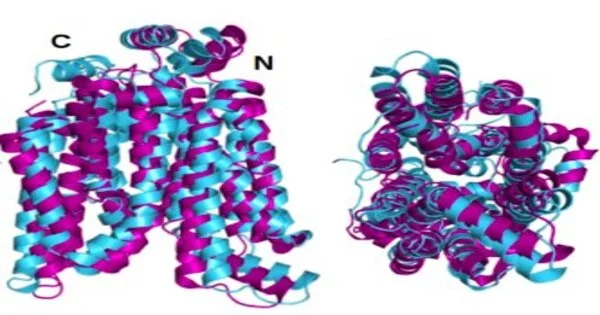
The glasslike or local state designs of two of the GLUTs—1 and 3—had already been uncovered by different analysts utilizing X-beam crystallography. To prove their own system, Dr. Zhang’s group initially anticipated the hydrophilic design of those two GLUTs by applying the QTY code’s amino corrosive substitutions and allowing AlphaFold2 to foresee the proteins’ shapes. It was as such, with incredible precision. The superposed hydrophobic and hydrophilic pictures are almost indistinguishable.
The group followed up this affirmation by joining QTY code and AlphaFold2 to foresee the hydrophilic designs of the 12 different GLUTs in record time. “In 2018, it required four to five weeks utilizing a fast PC group to mimic any protein structure,” says Dr. Zhang. “Presently, with AlphaFold2, we can utilize a Google PC for nothing, and it mimics the transmembrane proteins in hours.” A few little proteins took under 60 minutes. “
“This concentration on human film glucose carriers and their water-solvent QTY variations from the Zhang lab at MIT is entrancing,” says Professor Joel Sussman of the Weizmann Institute of Science in Rehovot, Israel, who was not engaged with the work. “It gives trial perception through X-beam crystallography and AI expectation utilizing AlphaFold2 to see, interestingly, at nuclear goal, the distinctions between hydrophobic ‘water-loathing’ helices and hydrophilic ‘water-adoring’ helices.” It is a basic move toward utilizing the QTY code strategy to study multispan transmembrane proteins and other collected proteins through their water-solvent variations and is probably going to have a huge effect in the area of biotechnology. “
MIT: Professor Robert Langer, whose work in biomedical design is profoundly celebrated, says, “The [QRB Discovery] paper is great, and I think it can possibly help a ton of disease patients.” Professor Langer was not engaged with the exploration.
The authors of the QRB Discovery paper are Dr. Zhang and Smorodina; Fei Tao and Rui Qing of Shanghai Jiaotong University (Dr. Qing was previously a postdoctoral specialist in the MIT Media Lab and later an examination researcher at the Koch Institute for Integrative Cancer Research at MIT); Dr. Steve Yang, an MIT graduate now at PT Metiska Farma in Indonesia; and Dr. David Jin, M.D., Ph. Avalon GloboCare likewise financed the exploration.
Dr. Jin says he trusts — yet this isn’t essential for the flow study — that future examinations will actually want to hereditarily change the glucose entry films to foster novel remedial targets.
“Presently, our main decisions for disease treatment are a medical procedure, chemo, or little particle treatment,” says Dr. Jin. “In the future, it could be feasible to take a patient’s T-cell, a part of the safe framework, and hereditarily change it in the lab so it can work practically like a disease looking through the GPS framework with a growth going after capacity.”
More information: Eva Smorodina et al, Comparing 2 crystal structures and 12 AlphaFold2-predicted human membrane glucose transporters and their water-soluble glutamine, threonine and tyrosine variants, QRB Discovery (2022). DOI: 10.1017/qrd.2022.6