Applications that rely on converting light into energy could benefit from an improved method for developing semiconductor materials at very small scales. A Los Alamos-based research group consolidated attractive dopants into extraordinarily designed colloidal quantum dabs—nnanoscale-size semiconductor precious stones—aand had the option to accomplish impacts that might control sunlight-based cell innovation, photograph locators, and applications that rely upon light to drive compound responses.
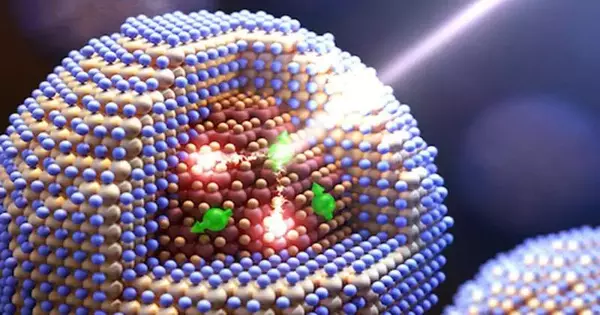
“In quantum specks containing a lead-selenide center and a cadmium-selenide shell, manganese particles go about as little magnets whose attractive twists unequivocally collaborate with both the center and the shell of the quantum spot,” said Victor Klimov, head of the Los Alamos nanotechnology group and the venture’s vital examiner. “Over these connections, energy can be moved to and from the manganese particle by flipping its twist—aa cycle usually named turn trade.”
A single absorbed photon causes an excited manganese ion to spin-flip relax, resulting in not one but two electron-hole pairs, also known as excitons, in spin-exchange carrier multiplication.
Because of the very quick pace of twist trade collaborations, the attractively doped quantum spots show a three-overlap upgrade in the transporter duplication yield compared with comparably organized un doped quantum dabs. Critically, the improvement is particularly huge in the scope of photon energies inside the sun-powered range, prompting conceivable photo conversion innovation applications.
“To perform spin-exchange carrier multiplication, properly engineered quantum dots are required. These dots’ bandgap must be less than half the energy of the manganese spin-flip transition, and their spin structure must match that of the excited manganese ion.”
Clement Livache, postdoctoral researcher and spectroscopy expert on the nanotechnology team.
The advantages of carrier multiplication
Normally, when a photon is absorbed by a semiconductor, it creates a “hole” in the valence band and an electron in the conduction band. This cycle underlies the activity of photodiodes, picture sensors, and sun-oriented cells, wherein the produced charge transporters are extricated as a photocurrent. In chemistry, photo generated electrons and holes can facilitate so-called redox reactions, which involve the transfer of electrons from one entity to another.
Carrier multiplication, which is triggered by a photon with high energy and results in the generation of a “hot” carrier with high kinetic energy, would be beneficial to all kinds of photoconversion schemes. After that, the electron is excited in the conduction band, and this energy is released when it collides with a valence-band electron. Consequently, a brand-new pair of electrons and holes is added to the original pair that was created by the absorbed photon.
In bulk solids, carrier multiplication is ineffective due to competing energy losses caused by interactions with lattice vibrations (commonly referred to as phonons). However, this effect was enhanced by chemically synthesized colloidal quantum dots, as Los Alamos researchers demonstrated for the first time in 2004. The tiny size of colloidal quantum specks builds the recurrence of electron impacts and, in this manner, works with transporter augmentation.
Nonetheless, even in the quantum spots, the productivity of transporter duplication isn’t sufficiently high to considerably affect the presentation of viable photoconversion plans. On account of mass-produced precious stones, the essential limit is energy misfortunes because of the quick emanation of phonons, prompting “ineffective” warming of a gem grid.
Turn trade collaborations to support transporter increases.
Manganese dopants assist with handling the issues of quick phonon emanation. Working off past exploration that exhibited the sub-picosecond timescales of twist trade collaborations—which are quicker than phonon emanation—the analysts understood that utilizing these associations would support the productivity of transporter augmentation.
“To order turn-trade transporter augmentation, one requires appropriately designed quantum specks,” said Lenient Livache, a postdoctoral specialist and spectroscopy master in the nanotechnology group. “In addition, the quantum dots’ spin structure must match that of the excited manganese ion, and their bandgap must be less than half of the energy of the manganese spin-flip transition.”
The project’s lead chemist, Hin Jo, stated, “The energy conditions can be satisfied with manganese-doped quantum dots containing a lead-selenide core and cadmium-selenide shell.” In these designs, transporter duplication happens through two twist-trade steps. To start with, the energy of the electron-opening pair, created by a consumed photon in the cadmium-selenide shell, is transferred to the manganese particle. Then, the manganese particle goes through a turn-flip unwinding back to the unexcited state by making two excitons in the number one spot in the selenide center.”
Turn-trade transporter duplication can be particularly helpful in multi-electron or opening responses that require numerous decrease and oxidation occasions. One of the bottlenecks in this situation is the standby time between consecutive decrease and oxidation steps. By producing pairs of charge carriers—two electrons and two holes—colocalized in both the temporal and spatial domains, carrier multiplication removes this obstruction.
Nature Materials is the journal that published the research.
More information: Ho Jin et al, Spin-exchange carrier multiplication in manganese-doped colloidal quantum dots, Nature Materials (2023). DOI: 10.1038/s41563-023-01598-x