Scientists from the Barts Disease Foundation (BCI) at Sovereign Mary College of London, the Italian Organization for Genomic Medication, and the College of Milan recognized a clever part for a malignant growth-causing quality in controlling a significant hereditary cycle that supports hereditary variety in prostate disease.
The discoveries, published today in Cell Reports, uncover what the quality means for the age of hereditary variations in prostate malignant growth that might anticipate illness backslide and address new medication focuses to work on quiet endurance.
Co-senior creator Dr. Prabhakar Rajan, Gathering Pioneer at BCI and Expert Urological Specialist at Barts Wellbeing NHS Trust, says that “prostate disease is the [most common] male malignant growth on the planet and the leading reason for male disease-related demise. It is truly reflected in its hereditary cosmetics, which makes finding and treatment precarious, as there isn’t a one-size-fits-all methodology for treating patients. Information on the drivers of hereditary fluctuation will assist us with understanding the illness better and further developing medicines. “
“This is a first for a controller of alternative splicing, and it could suggest that FOXA1 drives prostate cancer cells to operate in a way that is deleterious to patients.”
Dr. Prabhakar Rajan, Group Leader at BCI and Consultant Urological Surgeon at Barts Health NHS
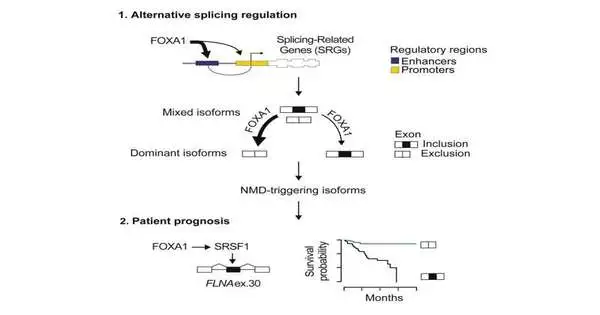
Elective joining is the cycle by which portions of qualities are rearranged to make various mixes of hereditary code known as “graft variations,” which give the directions to make proteins. Through elective joining, a solitary quality can code for numerous different proteins that are communicated at various levels and have different capabilities in the cell.
Elective joining is an important cycle for managing quality articulation and creating hereditary and protein variety inside normal cells; however, it is disrupted in a variety of disease types, including prostate cancer.
In this review, the group recognized that the malignant growth-causing quality FOXA1 is a vital controller of elective joining in prostate disease and may control the age of graft variations that impact illness backslide and patient endurance.
FOXA1 tweaks elective joining in prostate disease.
FOXA1 is a sort of protein known as a “trailblazer record factor. Record elements can choose which qualities in DNA are translated into the guidelines used to make proteins inside our cells and the rate at which this happens. As a trailblazer factor, FOXA1 opens up DNA for restriction by particular record factors. Changes to FOXA1 have been found to drive the inception and progression of prostate disease.
By surveying elective joining in cell line models and essential instances of prostate disease, the group found that elevated degrees of FOXA1 restricted hereditary variety towards graft variations that have a useful advantage for malignant growth cells. The examinations uncovered that FOXA1 leaned toward join variations that were available at undeniable levels inside the cells and hushed graft variations communicated at low levels, hence lessening the joining fluctuation in prostate disease.
(Dr. That’s what Rajan says. “This novel finding has never been displayed before for a regulator of elective joining and may imply that FOXA1 guides prostate disease cells to act with a certain goal in mind that might be impeding to patients.”
Co-senior creator Teacher Matteo Cereda, academic partner at the College of Milan and Gathering Pioneer at the Italian Foundation for Genomic Medication, added that “interestingly, we show that an early player of record guideline is likewise liable for the tweaking of elective joining.”
Possibly new treatment focuses
To decide if FOXA1-controlled elective joining affected patient endurance, the group examined clinical information from nearly 300 patients with essential prostate disease, accessible through The Malignant Growth Genome Map book.
Although elevated degrees of FOXA1 decreased joining inconstancy, the group found that FOXA1 improved the consideration of hereditary sections into graft variations that are solid markers of prostate disease repeat. The group uncovered that the consideration of one specific hereditary section in the join variation of a quality called the FLNA quality, which is constrained by FOXA1, gave a development benefit to prostate malignant growth cells, which might drive early illness backslide.
(Dr. Rajan says, “This study shows how we can take advantage of the force of genomics to make significant logical revelations about how hereditary fluctuation in prostate disease is controlled. We trust our discoveries will have clinical effect by recognizing more exact markers of illness and new potential medication targets. “
The group might now want to further test whether the genetic variations they have recognized to be connected to malignant growth repeat are helpful in foreseeing illness backslide and to embracing trials to decide if focusing on these qualities could address better approaches to treating prostate disease.
More information: Marco Del Giudice et al, FOXA1 regulates alternative splicing in prostate cancer, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.111404
Journal information: Cell Reports