In the competition to draw down ozone-depleting substances outflows all over the planet, researchers at MIT are focusing on carbon-catch advancements to decarbonize the most obstinate modern producers.
Steel, concrete, and substance fabrication are particularly troublesome ventures to decarbonize, as carbon and non-renewable energy sources are intrinsic to their creation. Innovations that can catch fossil fuel byproducts and convert them into structures that input into the creation interaction could assist with diminishing the general emanations from these difficult-to-subside areas.
In any case, hitherto, exploratory advances that catch and convert carbon dioxide do so in two separate cycles, which themselves require a colossal measure of energy to run. The MIT group is hoping to consolidate the two cycles into one integrated and undeniably more energy-efficient framework that might actually run on sustainable power to both catch and convert carbon dioxide from concentrated, modern sources.
“We discovered that reacting this’solo’ CO2 is easier than reacting CO2 that has been captured by the amine. This indicates to future researchers that this approach may be possible for industrial streams where high amounts of CO2 can be successfully absorbed and transformed into useful chemicals and fuels.”
Lead author and postdoc Graham Leverick
In a concentrate just distributed in ACS Catalysis, the specialists uncover the secret workings of how carbon dioxide can be both caught and changed over through a solitary electrochemical cycle. The cycle includes utilizing a terminal to draw in carbon dioxide let out of a sorbent and change it into a decreased, reusable structure.
Others have announced comparative showings, yet the components driving the electrochemical response have remained muddled. The MIT group completed broad tests to confirm that driver and found that eventually, it boiled down to the halfway strain of carbon dioxide. All in all, the more unadulterated carbon dioxide that connects with the anode, the more proficiently the cathode can catch and change the atom.
Information on this principal driver, or “dynamic species,” can help researchers tune and streamline comparable electrochemical frameworks to catch and change carbon dioxide in a coordinated cycle effectively.
The review’s outcomes infer that while these electrochemical frameworks would most likely not work for extremely weaken conditions (for example, to catch and change over fossil fuel byproducts straightforwardly from the air), they would be appropriate to the exceptionally focused emanations produced by modern cycles, especially those that have no undeniable inexhaustible other option.
“We can and ought to change to renewables for power creation. In any case, profoundly decarbonizing ventures like concrete or steel creation is testing and will take a longer time,” says concentrate on creator Betar Chivalrous, the Class of 1922 Vocation Improvement Academic Administrator at MIT. “Regardless of whether we dispose of all our power plants, we want a few answers for managing the discharges from different businesses in the longer term, before we can completely decarbonize them. That is where we see a perfect balance, where something like this framework could fit.”
The review’s MIT co-creators are lead creator and postdoc Graham Leverick and graduate understudy Elizabeth Bernhardt, alongside Aisyah Illyani Ismail, Jun Hui Regulation, Arif Arifutzzaman, and Mohamed Kheireddine Aroua of Sunway College in Malaysia.
Breaking bonds
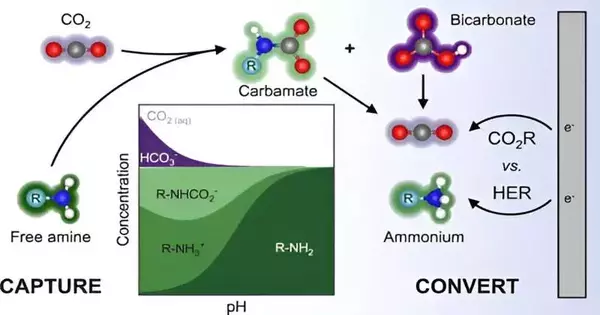
Carbon-catch advancements are intended to catch emanations, or “vent gas,” from the smokestacks of power plants and assembly offices. This is done principally by utilizing huge retrofits to pipe emanations into loads loaded up with a “catch” arrangement—a blend of amines, or smelling salt-based compounds, that synthetically tie with carbon dioxide, delivering a steady structure that can be isolated from the remainder of the vent gas.
High temperatures are then applied, ordinarily as non-renewable energy sources produce steam, to let the caught carbon dioxide out of its amine bond. In its unadulterated structure, the gas can then be siphoned into capacity tanks or underground, mineralized, or further changed over into synthetics or fills.
“Carbon capture is a developed innovation in that the science has been known for around 100 years; however, it requires truly enormous establishments and is very costly and energy-concentrated to run,” Heroic notes. “What we need are advancements that are more secluded and adaptable and can be adjusted to additional different wellsprings of carbon dioxide. Electrochemical frameworks can assist with tending to that.”
Her gathering at MIT is fostering an electrochemical framework that both recuperates the caught carbon dioxide and converts it into a reduced, usable item. She says a coordinated framework instead of a decoupled one could be completely controlled with inexhaustible power as opposed to a non-renewable energy source determined by steam.
Their idea focuses on a cathode that would squeeze into existing carbon-catch arrangements. At the point when a voltage is applied to the cathode, electrons stream onto the receptive type of carbon dioxide and convert it to an item utilizing protons provided by water. This makes the sorbent accessible to trap more carbon dioxide instead of utilizing steam to do likewise.
Heroic recently exhibited this electrochemical interaction that could attempt to catch and change carbon dioxide into a strong carbonate structure.
“We showed that this electrochemical cycle was achievable with early ideas,” she says. “From that point forward, there have been different examinations centered around utilizing this interaction to endeavor to deliver valuable synthetics and energizes. Yet, there have been conflicting clarifications of how these responses work in the engine.”
Solo CO2
In the new review, the MIT group used an amplifying glass in the engine to coax out the particular responses driving the electrochemical cycle. In the lab, they produced amine arrangements that look like the modern catch arrangements used to remove carbon dioxide from pipe gas.
They deliberately modified different properties of every arrangement, like the pH, fixation, and kind of amine, then ran every arrangement past a cathode produced using silver—a metal that is generally utilized in electrolysis studies and known to switch carbon dioxide over completely to carbon monoxide productively. They then, at that point, estimated the centralization of carbon monoxide that was changed over toward the end of the response and looked at this number against that of each and every other arrangement they tried to see which boundary had the most impact on how much carbon monoxide was delivered.
Eventually, they found that what made the biggest difference was not the kind of amine used to at first catch carbon dioxide, as many have thought. All things being equal, it was the grouping of solo, free-drifting carbon dioxide particles, which tried not to bond with amines, which were nevertheless present in the arrangement. This “solo-CO2” determined the convergence of carbon monoxide that was eventually created.
“We saw that it’s more straightforward to respond to this ‘solo’ CO2 when contrasted with CO2 that has been caught by the amine,” Leverick offers. “This lets future specialists know that this interaction could be practical for modern streams, where high convergences of carbon dioxide could productively be caught and changed over into helpful synthetics and energizers.”
“This isn’t an evacuation innovation, and it’s critical to express that,” Heroic burdens. “The value that it brings is that it permits us to reuse carbon dioxide some number of times while supporting existing modern cycles for less related discharges. At last, my fantasy is that electrochemical frameworks can be utilized to work with mineralization and superdurable stockpiling of CO2—a genuine expulsion innovation. That is a more drawn-out vision. What’s more, a ton of the science we’re beginning to comprehend is an initial move toward planning those cycles.”
More information: Graham Leverick et al, Uncovering the Active Species in Amine-Mediated CO2 Reduction to CO on Ag, ACS Catalysis (2023). DOI: 10.1021/acscatal.3c02500