The review distinguished the adverse results of upsetting meiosis.
Another concentration in a creature model of maturing demonstrates a possible justification for why ladies who have early menopause or other hereditary circumstances influencing the conceptual framework are more inclined to foster cardiovascular sickness, diabetes, and dementia.
The new study, led by scientists from the University of Pittsburgh and UPMC and published in the journal Maturing Cell, discovered that disrupting a cycle known as meiosis in C. elegans regenerative cells caused a decrease in the worms’ wellbeing and triggered a sped-up maturing quality mark similar to that of maturing people.
“This review is energizing since the main direct proof controlling the wellbeing of conceptive cells prompts untimely maturing and a decrease in healthspan,” said senior author Arjumand Ghazi, Ph.D., academic administrator of pediatrics, formative science, and cell science and physiology at Pitt and UPMC Youngsters’ Medical Clinic of Pittsburgh. “The implications of this finding are significant: it suggests that the situation with the conceptual framework is important not only for creating children, but also for overall well-being.”
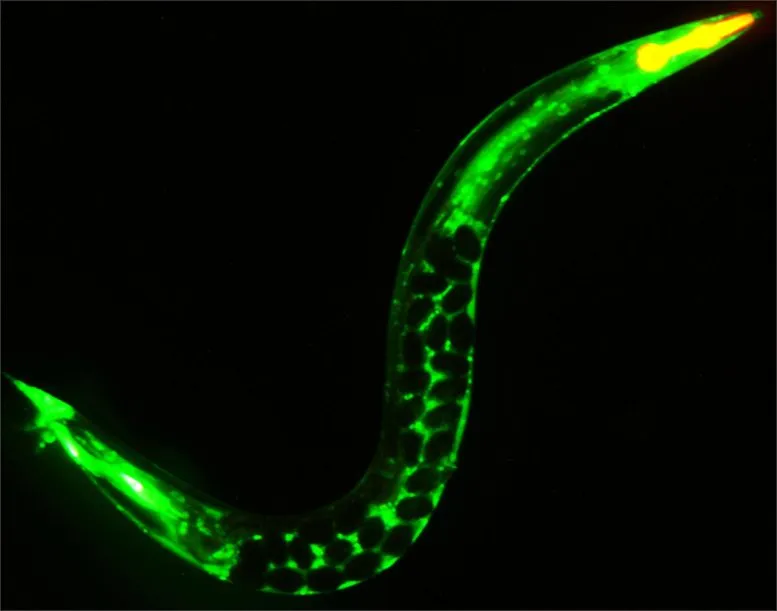
C. elegans, a youthful grown-up worm
A young C. elegans grown-up and sparkling green where a protein has been linked to a fluorescent tag and loaded up with eggs that will be laid as dim circles in the mother’s body.Interruption of meiosis, a cycle on which the formation of these eggs depends, abbreviates the creature’s whole life expectancy and speeds up its maturing. Credit: Scott Keith and Arjumand Ghazi
While the results of maturing on ripeness are notable, research in the past twenty years has begun to show that conceptive wellness likewise affects human maturing and wellbeing. The issue is that it is difficult to look at this type of situation and the logical outcomes in people right away.Ghazi and her colleagues then moved on to Caenorhabditis elegans, a tiny nematode worm that is ideal for maturing research due to its short lifespan (three weeks from birth to death) and ability to transmit hereditary pathways to humans.

Arjumand Ghazi, Ph.D., is the academic partner of pediatrics, formative science, and cell science and physiology at the College of Pittsburgh and the UPMC Youngsters’ Medical Clinic of Pittsburgh. Credit: UPMC
The scientists concentrated on meiosis, a sort of cell division present in all creatures from yeast to people that happens solely in cells bound to deliver sperm or eggs. They found that creatures with changes in meiosis qualities had more limited lives than their non-transformed partners. The freaks also had more negative overall health evaluations, including untimely decreases in portability, strong capability, and memory.
“The interesting piece of this lifespan work was that these creatures likewise gave indications of upset protein homeostasis,” said Ghazi. “A disruption in the equilibrium of proteins within cells is at the heart of related neurodegenerative infections, such as Alzheimer’s disease.”
At the point when the specialists further developed protein homeostasis in the worms, some deficiency in life expectancy was forestalled. These discoveries highlight upset proteostasis as a key component connecting conceptual wellbeing and maturing.
Then, the group took a gander at quality articulation changes in C. elegans. At day 1 of adulthood, meiosis freaks communicated qualities that were strikingly similar to those that typical worms wouldn’t communicate until day 10.
“In human terms, it resembles somebody in their mid-20s having the actual appearance, physiology, and quality marks of a 70-year-old,” made sense of Ghazi. “Playing with meiosis decisively affects healthspan and speeds up maturing in C. elegans.”
A significant number of similar qualities control the maturation of worms and people. So the scientists wondered if the quality mark of meiosis freaks resembled the qualities of maturing people.They discovered that this was, indeed, the case—a remarkable discovery because it suggests that disrupting the regenerative framework may result in comparative changes from worms to humans.
Because C. elegans can be used to render central disclosures impractical in humans and more complex frameworks, this discovery opens up extraordinary opportunities for understanding how the conceptual framework shapes maturing, according to Ghazi.
She is currently looking to collaborate with UPMC Magee-Womens Emergency Clinic and Magee-Womens Exploration Organization to further test this hypothesis in human patients who, due to hereditary illness, experience premature menopause and exhibit complications such as coronary illness, immune system problems, and osteoporosis.
“Informed by our work in C. elegans, we need to cultivate a board with old enough related qualities and use this to screen patients’ blood and spit,” Ghazi explained.”In the event that we see proof of similar qualities being raised in patients, it would be a significant initial move toward stretching out such examinations to ladies who go through early menopause and early barrenness.”
Ghazi believes that in the long run, this research could lead to tests for early detection of wellbeing impediments caused by conceptual irregularities, as well as new medications or the repurposing of existing medications to treat such age-related illnesses.
Reference: “Meiotic dysfunction accelerates somatic aging in Caenorhabditis elegans” by Julia A. Loose, Francis R. G. Amrit, Thayjas Patil, Judith L. Yanowitz and Arjumand Ghazi, 29 September 2022, Aging Cell.
DOI: 10.1111/acel.13716





