Researchers have discovered how gram-negative bacteria, which cause drug-resistant pneumonia, bloodstream infections, and surgical site infections in hospitalized patients, complete the construction of an important component of their outer membrane that protects these pathogens from immune system and antibiotic attacks. The new findings could hasten the development of new medications to combat these potentially lethal germs, which cause innumerable infections in health-care settings around the world.
The report was published online on April 6th by Nature.
Previously, we knew that gram-negative bacteria build their outer membranes from two main non-protein components: lipids and carbohydrates, which together form an impermeable barrier. The missing link was how this lipopolysaccharide component came together. ” Filippo Mancia, Ph.D., a co-leader of the study and professor of physiology and cellular biophysics at Columbia University Vagelos College of Physicians and Surgeons, agrees.
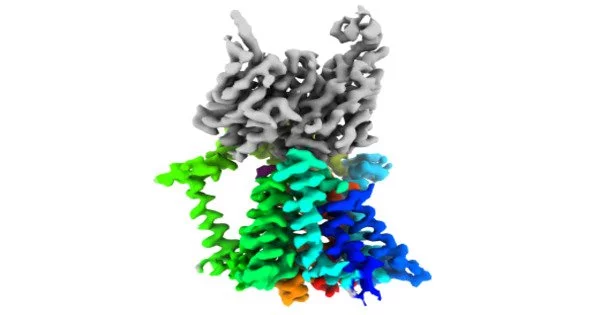
Mancia and colleagues used cutting-edge single-particle cryo-electron imaging to establish the structures of the enzyme that connects lipids and sugars (called an O-antigen ligase) in two different functional configurations. The scientists then learned how the enzyme places the lipids and carbohydrates so that they may join together to form the protective membrane by combining genetic, biochemical, and molecular dynamics investigations. Gram-negative
“Previously, we knew that Gram-negative bacteria construct their outer membrane with two main, non-protein components—lipids and sugars—which together form an impermeable barrier. The missing link was how this lipopolysaccharide component comes together,”
Filippo Mancia, PhD,
bacteria rely on the lipopolysaccharide component of their outer membrane for survival. “If you could stop it from assembling, the bacteria would become more sensitive to medications and more vulnerable to the immune system,” Mancia explains.
The construction of this membrane is a continual process that begins with the formation of gram-negative bacteria and continues as the membrane naturally degrades and requires repair. “This means we’d have multiple chances to damage the membrane, not just at one point in the bacteria’s life cycle,” Mancia explains.
After determining the structure of the enzyme responsible for the final and most important stage in the formation of lipopolysaccharide barriers in drug-resistant bacteria, the researchers may be able to develop medications that impede the production of this protective membrane.
Khuram U. Ashraf, PhD, who was a postdoctoral fellow in Mancia’s group at Columbia until recently, is the study’s lead author. M. Stephen Trent of the University of Georgia in Athens, GA, and Phillip J. Stansfeld of the University of Warwick in Coventry, UK, are the other study co-leaders. Other Columbia researchers who contributed significantly to this work included Rie Nygaard, PhD, Oliver Clarke, PhD, Anne-Catrin Uhlemann, MD, and Thomas McConville, MD.”Structural
basis of lipopolysaccharide maturation by the O-antigen ligase was established. ” Owen N. Vickery (University of Warwick, Coventry, United Kingdom), Satchal K. Erramilli (University of Chicago, Chicago, IL), Carmen M. Herrera (University of Georgia, Athens, GA), Vasileios I. Petrou (Rutgers Biomedical Health Sciences, Newark, NJ), Sabrina I. Giacometti (Columbia), Meagan Belcher Dufrisne (Columbia), Michael Loukeris (New York Structural Biology Center, New York, NY), Brian Kloss (New York Structural Biology Center), Karolina Skorupinska-Tudek (Polish Academy of Sciences, Warsaw, Poland), Ewa Swiezewska (Polish Academy of Sciences), David I. Roper (Columbia and University of Warwick), Anthony A. Kossiakoff (Polish Academy of Sciences), David I. Roper (Columb (University of Chicago).
Grants from the National Institutes of Health (GM132120), AI150098, AI138576, AI129940, GM117372, GM116799, U54 DK104309, T32 AI100852, and K08 AI146284), Wellcome, the MRC, and the BBSRC supported the research.