Past neuroscience research reliably tracked down a connection between deviations from the “typical” iron digestion, otherwise called iron dysregulation, and different neurodegenerative infections, including Parkinson’s illness (PD) and Various Sclerosis (MS). In particular, cerebrum areas related with these illnesses have been viewed as frequently populated by microglia (i.e., occupant safe cells) loaded with Iron.
While the relationship between iron dysregulation and neurodegenerative sicknesses is irrefutably factual, the manners by which iron amassing influences the physiology of microglia and neurodegeneration are yet to be completely gotten a handle on. Scientists at worldwide medical organization Sanofi have as of late completed a review pointed toward filling this hole in the writing, by better comprehension how microglia answer iron.
“For quite a long time it has been realized that iron collects in impacted cerebrum districts in PD, MS and other neurodegenerative sicknesses,” Timothy Hammond, one of the scientists who did the review, told MedicalXpress. “This is the kind of thing we can find in patients utilizing X-ray imaging, where it has been shown that iron levels increment throughout the span of the illness. We additionally had our own information from moderate MS patients showing iron dysregulation in cerebrum microglia, the occupant safe cells of the mind.”
“This is something we can detect in patients using MRI imaging, where it has been demonstrated that iron levels rise over the course of the disease. We also have evidence from progressing MS patients that showed iron dysregulation in brain microglia, the brain’s resident immune cells.”
Timothy Hammond, one of the researchers who carried out the study,
The critical goal of the new work by Hammond and his partners was to more readily comprehend what iron amassing in microglia means for these cells’ working and wellbeing. Their work expands on their past examinations, and on the 2012 revelation of an iron-subordinate type of cell demise, known as ferroptosis.
Ferroptosis is a type of cell demise that is intervened by iron-subordinate lipid peroxidation, an interaction that harms lipids by oxidizing them. In their paper, the specialists’ guessed that iron-loaded microglia are helpless to ferroptosis and that this could assume a part in neurodegenerative sicknesses.
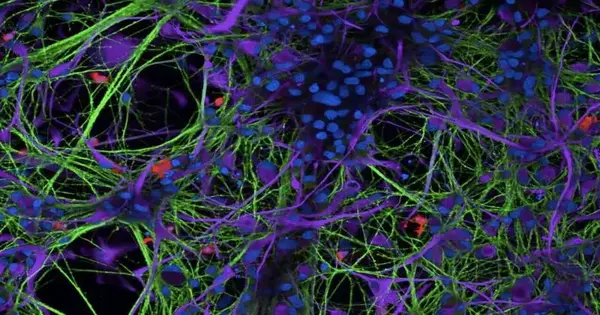
“We needed to use a few methodologies in this review, including single cell transcriptomics and CRISPR, however the device that truly permitted us to prod separated these systems was a complex tri-culture of human iPSC-determined cells containing microglia, astrocytes, and neurons — three of the significant cell types in the mind,” Hammond made sense of. “This instrument was recently evolved by a researcher in my group, Sean Ryan, who is likewise the lead creator of our paper.”
To lead their tests, the specialists developed microglia in a tri-culture framework. Utilizing a progression of hereditary and trial methods, they then showed that these microglia are exceptionally receptive to press and furthermore defenseless to ferroptosis.
Moreover, the group showed that an over-burden of iron causes a change in the microglial transcriptional state, which covers with a transcriptomic signature saw in microglia in mind tissue from perished patients with PD. At the point when they eliminated microglia from their tri-culture framework, Hammond and his associates saw that iron-actuated neurotoxicity in the framework altogether dialed back. This recommends that microglia reactions to press over-burden assume a urgent part in neurodegeneration.
This study is one of the first to show how iron-loaded microglia could add to various PD and other neurodegenerative infections. Later on, it could prepare for new significant disclosures, possibly illuminating the improvement regarding new helpful intercessions for these illnesses.
“We accept microglia are really trying safeguard neurons by taking up harmful iron and putting away it securely, yet over the long run the microglia become overpowered and bite the dust, delivering the put away iron and making the neurons kick the bucket as well,” Hammond said. “We must watch out for how we target microglia remedially, in light of the fact that they perform bunches of useful exercises, yet in the event that we can find a designated approach this could be a significant hub for illnesses like Parkinson’s with unnecessary iron collection.”
More information: Sean K. Ryan et al, Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration, Nature Neuroscience (2022). DOI: 10.1038/s41593-022-01221-3
Jonathan D. Proto et al, Disrupted microglial iron homeostasis in progressive multiple sclerosis, bioRxiv (2021). DOI: 10.1101/2021.05.09.443127