An exploration group from the LKS Workforce of Medication at the College of Hong Kong (HKUMed) found that physical cancellation of a growth silencer quality AKTIP advances luminal bosom disease improvement and protection from endocrine treatment. The discoveries are currently distributed in cell reports.
Bosom disease is the most common malignant growth and the third leading cause of death among women in Hong Kong, and it can be classified into a few sub-atomic subtypes. Each subtype has unmistakable clinical attributes, hereditary profiles, and treatment rules. While the disease can be genetic, with acquired changes in qualities such as BRCA1, the majority of breast cancer cases are significant and result from non-acquired transformations acquired during one’s lifetime.
The luminal bosom disease, which communicates a chemical receptor called estrogen receptor (ER), is the most well-known subtype and comprises 60–70% of all bosom malignant growth cases. Because disease cells express ER, which stimulates malignant growth improvement, focusing on ER by restorative specialists (endocrine treatment) is the foundation of the executives for luminal breast cancer.
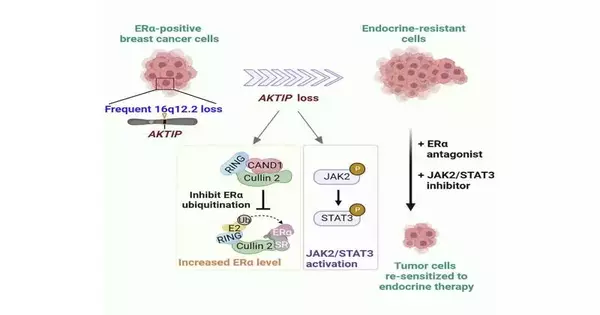
Regardless, 33% of luminal bosom disease patients who initially respond to endocrine treatment ultimately benefit from treatment resistance.Cancellation of the AKTIP quality is seen in around 55% of luminal bosom disease cases. Regardless of the great event, the result of the quality cancellation was obscure.
Through multi-omics and sub-atomic science approaches utilizing bosom disease cell lines, clinical examples, a mouse model, and malignant growth patient-inferred organoids, the group made an interesting revelation: deficiency of the AKTIP quality advances bosom disease through expanding the protein articulation level of ER. An examination of patient data revealed that luminal bosom disease patients with AKTIP-quality erasure have more terrible endurance, consistent with the favorable to malignant growth results seen in these trial models.
Significantly, breast cancer cells with AKTIP misfortune are impervious to endocrine treatment. This endocrine obstruction is because of the simultaneous enactment of another endurance pathway, JAK2/STAT3, which addresses an elective getaway pathway used by the disease cells when ER is hindered. Expanding on this discovery, the researchers discovered that inhibiting this alternative break pathway with the option of a JAK2/STAT3 inhibitor can overcome the opposition.
This study distinguished another driver distortion of luminal bosom malignant growth from the restorative prospects focusing on AKTIP quality erasure in bosom cancers.
“We present obvious proof that erasure of AKTIP is a prognostically and remedially significant chromosomal change in luminal bosom malignant growth.” Our findings that JAK2/STAT3 inhibitors can reverse the endocrine opposition caused by AKTIP deletion should be investigated further. Genomic information from growth DNA profiling is progressively directing disease care, in which customized treatment can be formed in light of the quality status of the malignant growth patient. This accurate medication approach can kill growth cells productively with fewer harmful secondary effects. “The fuse of AKTIP’s quality status as a prescient biomarker may refine the therapy technique for luminal bosom malignant growth,” made sense to Dr. Lydia Cheung Wai-chime, right-hand teacher of the School of Biomedical Sciences, HKUMed.
More information: Angel S.N. Ng et al, AKTIP loss is enriched in ERα-positive breast cancer for tumorigenesis and confers endocrine resistance, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.111821