Hydrogen could be a significant piece of our future energy supply: It can be put away, moved, and consumed depending on the situation. Be that as it may, the vast majority of the hydrogen accessible today is a result of flammable gas creation, and this needs to change for environmental security reasons. The best system such a long way to deliver harmless to the ecosystem “green hydrogen” is to partition water into hydrogen and oxygen, utilizing power that comes from environmentally friendly power sources, for instance, photovoltaic cells.
Nonetheless, it would be a lot simpler if daylight were utilized straightforwardly to part the water. This is precisely what new impetuses are now making possible, in a cycle known as “photocatalytic water parting.”The idea isn’t yet being utilized economically. At TU Wien, significant advances have now been steered toward this path: on a nuclear scale, researchers have understood another mix of sub-atomic and strong state impetuses that can finish the work while utilizing somewhat reasonable materials.
“Titanium oxide is light sensitive, which was already known. The energy of absorbed light causes the formation of free-moving electrons and positive charges in titanium oxide. These charges subsequently allow the clusters of atoms on this surface to help divide water into oxygen and hydrogen.”
Alexey Cherevan
Communication of molecules
“In reality, to have the option to part water with light, you need to tackle two undertakings simultaneously,” says Alexey Cherevan from the Institute for Materials Chemistry at TU Wien. We need to ponder oxygen and hydrogen. The oxygen particles of the water should be changed into O2 atoms, and the excess hydrogen particles—which are simply protons—should be transformed into H2 atoms.
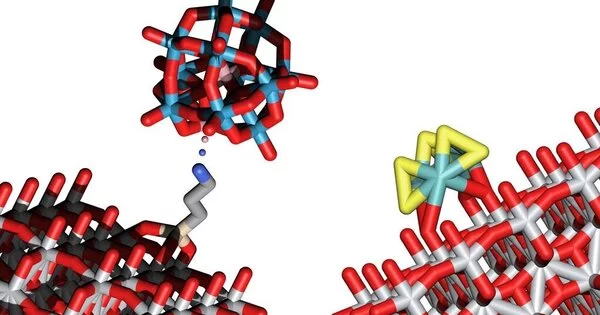
Arrangements have now been found for the two undertakings. Little inorganic groups comprising just a few molecules are secured on the surface of light-retaining support designs like titanium oxide. The blend of bunches and painstakingly picked semiconductor upholds leads to the ideal way of behaving.
The bunches responsible for oxidizing oxygen are comprised of cobalt, tungsten, and oxygen, while groups of sulfur and molybdenum are particularly reasonable for making hydrogen particles. The scientists at TU Wien were quick to store these bunches on a surface made of titanium oxide, where they could go about as impetuses for water parting.
“Titanium oxide is delicate to light, so that was at that point notable,” says Alexey Cherevan. “The energy of the retained light prompts the production of free-moving electrons and free-moving positive charges in the titanium oxide.” These charges then, at that point, permit the bunches of particles sitting on this surface to work with the parting of water into oxygen and hydrogen.
Exact control, iota by particle
Other research groups working on parting water with light depend on nanoparticles that can take on totally different shapes and surface properties. This makes sense to Alexey Cherevan. “The sizes are difficult to control; the molecules are not exactly organized similarly. Hence, in this situation, it is unimaginable to expect to make sense of precisely the way that the catalysis cycle happens exhaustively. ” At TU Wien, however, the specific construction of the still is up in the air with nuclear accuracy, which permits a full comprehension of the synergist cycle.
“This is the best way to get input on what the proficiency of the interaction truly relies upon,” says Alexey Cherevan. “We would rather not simply depend on an experimentation approach and attempt different nanoparticles until we see which one is the best one — we need to find out at the nuclear level what the ideal impetus truly is.”
Because the chosen materials have been shown to be effective at parting water, the next step is to fine-tune their careful construction to achieve significantly higher efficiencies.
Basic and promising.
“The conclusive benefit of our technique over parting water by electrolysis is its straightforwardness,” Alexey Cherevan stresses. Electric hydrogen creation first needs an economical energy source—like photovoltaic cells, perhaps an electric energy stockpiling gadget, and an electrolysis cell. With everything taken into account, this results in a generally complicated framework comprised of a huge number of natural substances. For photocatalytic water parting, then again, everything necessary is a reasonably covered surface that is covered by water and lighted by the sun.
In the long run, this strategy could be used to deliver more complicated particles by utilizing the concept of fake photosynthesis. It could be feasible to utilize the energy of sun-based radiation to deliver hydrocarbons with carbon dioxide from the air and water, which can then be utilized for different applications.
The related examinations show up in ACS Catalysis and ACS Materials Au.