Following a 10-year research focus on that began coincidentally and was met with doubt, a group of Northeastern College mechanical designers had the option to blend profoundly thick, super thin silicon nanowires that could upset the semiconductor business. Their exploration shows up in Nature Correspondences.
Northeastern mechanical and modern design professor Yung Joon Jung says it could have been his top exam project.
Jung, who works in the designing and use of nanostructure frameworks and recently concentrated on carbon nanotubes, says that “everything is new, and it requires a ton of diligence.”
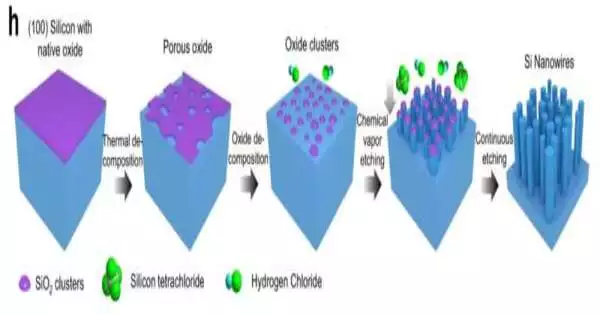
Jung and his colleagues, including another Northeastern mechanical design professor, Moneesh Upmanyu, have made significant progress in nanowire blend by discovering a new, profoundly thick type of silicon and mastering a new, versatile impetus-free carving cycle to create tiny silicon nanowires of two to five nanometers in width.
Understudies carried Jung’s regard for a strange consequence of a trial they were leading utilizing silicon wafers. Jung says the material he saw under an electron magnifying lens was not the same as the one they planned to create.
Jung chose to figure out more about this substance and found that it was silicon with “an extremely small” wire-like nanostructure, he says. They had the option to repeat the new material, he says, yet when they attempted to further develop the union cycle, the nanowires didn’t develop.
The researcher and his group needed to rewind and study all along the blend system and the material’s nuclear scale design and properties. Jung, an experimentalist, chose to enroll Upmanyu, who utilizes hypothesis, PC displaying, and recreation to grasp materials and make sense of tests.
“I generally need assistance from Moneesh to comprehend what’s going on,” Jung says.
The researchers felt that perhaps the substance coming about because of silicon wafers during union was not silicon by any means. The material had a profoundly packed structure, and the density decreased by 10% to 20% compared with ordinary silicon, which typically isn’t steady in a particularly compacted state, Upmanyu says.
A portion of their partners and exploration commentators were of a similar assessment. “They would agree, ‘This ought not be silicon’ or ‘This shouldn’t happen with silicon,'” Jung says.
Through the computational examination and display, Upmanyu had the option to show that, in spite of the strange properties, the new material was a type of silicon with a slim layer of oxide on top, which likely supports the pressure, he says.
“This material is extremely encouraging,” he says. “That pressure, I feel, is at the core of the multitude of fascinating properties you see.”
One reason silicon is broadly utilized as a semiconductor in microelectronics—for example, CPUs, coordinated circuits, semiconductors, silicon diodes, and fluid gem shows—is that it is modest and bountiful, Upmanyu says. As per the Regal Society of Science, it is the second most bountiful component in the world’s hull after oxygen, yet it doesn’t happen in its unadulterated, uncombined state in nature. It tends to be tracked down in sand, quartz, rock, rock, mica, and mud, among different stones and minerals.
During the 1970s, the flourishing silicon CPU industry even gave another name to the southern area of the San Francisco Cove — “Silicon Valley” — which was promoted by Wear Hoefler, an Electronic News Magazine columnist.
Nonetheless, customary silicon can’t endure high temperatures and, thus, is restricted to bringing down power applications. It has a bandgap of 1.11 eV (bandgap decides the energy expected to make the electrons in the semiconducting material lead power after being animated by outer sources).
The new material has a super wide bandgap of 4.16 eV—a world record, Jung says. The super wide bandgap infers that the material needs bigger boosts to lead power yet can work at high power, high temperature, and high frequencies. Jung says silicon nanowires created from this new material will be reasonable for power gadgets, semiconductors, diodes, and drive gadgets.
Dissimilar to normal silicon, the new material is profoundly impervious to oxidation. It is likewise photoluminant—ready to emanate blue and purple light, which can be utilized for bright lighting and in blue light diodes.
Jung and his examination group have likewise developed another strategy for creating silicon nanowires called compound fume drawing, which eliminates material as opposed to developing gems. Thus, they can make nanowires that are 10 to multiple times less expensive than the silicon nanowires right now being utilized monetarily.
Recently, known nanowire blend processes use impetus particles to develop silicon gems.
“The impetus free angle couldn’t possibly be more significantly significant, as it kills the need to eliminate the impetus after blend, which constantly debases the useful properties of the nanowires,” Jung says.
At times, impetus particles become pieces of the nanowire surface, he says, and their expulsion is beyond difficult.
Right now, the researchers can repeat nanowires with a controlled length of up to 100 microns.
“I feel a wide effect proceeding,” Upmanyu says. “This compound fume carving strategy that he [Jung] has spearheaded will be valuable for a large group of different materials…” You can imagine electronic applications or any application where you need to have a little size aspect of a material made…. It is strong.
He says that the new silicon material ought to be alluring for the semiconductor business. It tends to be utilized in military radios, radars, and photovoltaics like sun-based cells. Normal silicon bandgap doesn’t permit handling bright light and using it for creating power, Upmanyu says.
“Thus, in the event that you have a wide-bandgap material, which is modest, plentiful, similar to silicon, you can currently have high-proficiency sun-based cells,” he says.
It is even used to harvest sun-based energy that has been submerged.Water ingests the red and infrared range, Upmanyu says, so sun-based cells that can reap blue and bright light become vital.
The new silicon nanowires can further develop lithium particle batteries, Jung says. Upmanyu says that further adding a few select materials like phosphorus or nitrogen (a method called doping) can prompt other intriguing properties and permit different applications.
He accepts that different fascinating quantum peculiarities can be controlled in these silicon nanowires because of their tiny size, which makes this material promising for quantum data handling and perhaps quantum figuring, Upmanyu says.
A few other design foundations all over the planet added to this exploration, including Korea Organization of Science and Innovation, Korea Progressed Establishment of Science and Innovation, Tokyo College of Science, College of Science and Innovation of China, and Rensselaer Polytechnic Foundation in New York.
The exploration isn’t finished. The researchers are keen on understanding all the science behind the cycle and sorting out why the pressure of this type of silicon is so steady. They need to improve the carving system to create a smoother surface and further scale it up for modern applications.
“You need to have the option to comprehend the cycle so you can control it to do what you need to do,” Upmanyu says.
They will be searching for partners keen on making gadgets with this new silicon material.
“You need another type of something you made to be taken on as broadly as could be expected. “I think commercialization and gadget mix are the keys here,” Upmanyu says.
More information: Sen Gao et al, Catalyst-free synthesis of sub-5 nm silicon nanowire arrays with massive lattice contraction and wide bandgap, Nature Communications (2022). DOI: 10.1038/s41467-022-31174-x
Journal information: Nature Communications