Dr. Benjamin Rotstein and partners reveal a functionally basic strategy to plan carbon isotope-named forms of medications and diagnostics.
The advancement of new drugs depends on the capacity of researchers to plan rich, unambiguous medications for designated clinical preliminary examinations. Also, the isotopic naming of medication applicants in research labs is vital in this general effort.
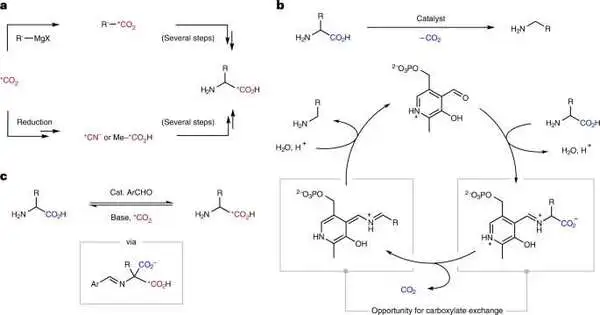
In another review, Dr. Benjamin Rotstein’s lab at the University of Ottawa School of Medicine has teamed up with partners to reveal a functionally basic strategy to plan carbon isotope-named forms of medications and diagnostics. They fostered a strategy to trade a solitary iota in amino acids of Ottawa School of Medicine has teamed up with partners to reveal a functionally basic strategy to plan carbon isotope-named forms of medications and diagnostics. They fostered a strategy to trade a solitary iota in amino acids—the building blocks of proteins that are likewise used to plan particles—for its isotope.
“This is critical in drug development because we want to know where the medication goes in the body, how it is metabolized, and how it is removed so we can design proper dose and toxicity trials,”
Dr. Rotstein, an associate professor in the Faculty of Medicine’s Department of Biochemistry, Microbiology and Immunology.
“This is truly significant in drug improvement since we need to know where the medication goes in the body and how it could be used and killed so we can design proper dosing and harmfulness studies,” says Dr. Rotstein, an academic partner in the Department of Medication’s Branch of Natural Chemistry, Microbial Science, and Immunology.
The work was depicted in a paper in Nature Science.
Dr. Rotstein’s lab initially planned their tests to function as an impetus that our bodies use: pyridoxal phosphate, which removes carboxylic corrosive from amino acids and is a dynamic type of vitamin B-6.Yet, he says, they needed to make it run backward, and it turned out the system was somewhat different from what they had anticipated at first.
“We’re actually adding carbon dioxide and then removing the corrosive.””So an alternate system permits us to think about far better impetuses and growing the degree further past amino acids,” he says.
The exploration was finished as a team with College of Alberta partners and physicists at Sanofi, the French drug organization. Dr. Rotstein’s lab did the carbon-11 examinations and worked with these partners to reveal the system of the response. His lab utilizes carbon-11 since radioactive material functions admirably for clinical imaging.
Dr. Rotstein and his group are currently concentrating on the best way to make the response produce only one “perfect representation” form of amino acids so analysts won’t have to isolate them afterward.
He says they are particularly excited about utilizing carbon-11 amino acids to gauge the rate at which our bodies are creating proteins since this can be a mark of illness.
“We’re also using these in imaging studies right now to figure out digestion and protein blend rates in various tissues,” says Dr. Rotstein, who also heads the College of Ottawa Heart Foundation’s subatomic imaging tests and radiochemistry lab.
More information: Odey Bsharat et al, Aldehyde-catalysed carboxylate exchange in α-amino acids with isotopically labelled CO2, Nature Chemistry (2022). DOI: 10.1038/s41557-022-01074-0
Journal information: Nature Chemistry