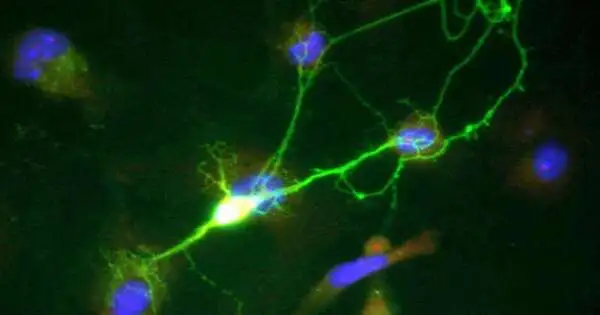
Another Northwestern medication concentrates on difficulties and a typical lack of confidence in what sets off Parkinson’s illness.
The degeneration of dopaminergic neurons is generally acknowledged as the primary cause of Parkinson’s. However, the new review recommends that a brokenness in the neuron’s neurotransmitters—the little hole across which a neuron can send a drive to another neuron—prompts shortages in dopamine and goes before the neurodegeneration.
Parkinson’s infection influences 1% to 2% of the populace and is characterized by a resting quake, unbending nature, and bradykinesia (gradualness of development). These engine side effects are because of the dynamic loss of dopaminergic neurons in the midbrain.
The review, “Parkinson’s illness-connected parkin transformation upsets reusing of synaptic vesicles in human dopaminergic neurons,” distributed in Neuron, opens another road for treatments, the researchers said.
“We showed that dopaminergic neurotransmitters become useless before neuronal passing happens,” said lead creator Dr. Dimitri Krainc, seat of nervous system science at Northwestern College Feinberg Institute of Medication and head of the Simpson Querrey Community for Neurogenetics.
“In light of these discoveries, we speculate that focusing on broken neurotransmitters before the neurons are declined may address a superior remedial methodology.”
The review examined patient-determined midbrain neurons, which is basic since mouse and human dopamine neurons have an alternate physiology and discoveries in the mouse neurons are not translatable to people, as featured in Krainc’s examination distributed in Science.
Northwestern researchers found that dopaminergic neural connections are not working accurately in different hereditary types of Parkinson’s illness. This work, along with other late examinations by Krainc’s lab, addresses one of the significant holes in the field: how various qualities connected to Parkinson’s lead to the degeneration of human dopaminergic neurons.
Neuronal reusing plant
Imagine two specialists in a neuronal reusing plant. They must reuse mitochondria, the energy makers of the cell, that are excessively old or exhausted. On the off chance that the useless mitochondria stay in the cell, they can cause cell breakdown. T
The cycle of reusing or eliminating these old mitochondria is called mitophagy. The two specialists in this reuse system are Parkin and Pink1. In a typical circumstance, PINK1 enacts Parkin to move the old mitochondria to be reused or discarded.
It has been deeply grounded that individuals who convey transformations in the two duplicates of either PINK1 or Parkin foster Parkinson’s illness as a result of incapable mitophagy.
The account of two sisters whose sickness helped advance Parkinson’s examination
Two sisters had the misfortune of being brought into the world without the PINK1 quality, on the grounds that their parents were each missing a duplicate of the basic quality. This put the sisters at high risk for Parkinson’s illness, yet one sister was analyzed at age 16, while the other was not analyzed until she was 48.
The justification for the dissimilarity prompted a significant new revelation by Krainc and his gathering. The sister who was analyzed at 16 likewise had fractional loss of Parkinson’s, which, without anyone else, shouldn’t cause Parkinson’s.
“There should be a complete loss of Parkin to cause Parkinson’s sickness. In this way, for what reason did the sister with just a fractional loss of Parkin get the sickness over 30 years sooner?” Krainc inquired.
Thus, the researchers understood that Parkin has another significant work that had recently been obscured. The quality likewise works in an alternate pathway in the synaptic terminal, irrelevant to its reusing work, where it controls dopamine discharge. With this new comprehension of what turned out badly for the sister, Northwestern researchers saw another chance to help Parkin and the possibility of forestalling the degeneration of dopamine neurons.
“We found another system to actuate Parkin in persistent neurons,” Krainc said. “Presently, we want to foster medications that animate this pathway, correct synaptic brokenness, and ideally forestall neuronal degeneration in Parkinson’s.”
The principal creator of the review is Pingping Melody, a research associate teacher in Krainc’s lab. Different creators are Wesley Peng, Zhong Xie, Daniel Ysselstein, Talia Krainc, Yvette Wong, Niccol Mencacci, Jeffrey Savas, and D. James Surmeier from Northwestern, and Kalle Gehring from McGill College.
More information: Dimitri Krainc et al, Parkinson’s disease linked parkin mutation disrupts recycling of synaptic vesicles in human dopaminergic neurons, Neuron (2023).