When compared to fossil fuels, nuclear power is typically regarded as a less polluting method of power generation. It does not produce byproducts like carbon dioxide or other air pollutants or greenhouse gases. However, it generates radiotoxic waste that must be treated appropriately to avoid adverse health and environmental conditions.
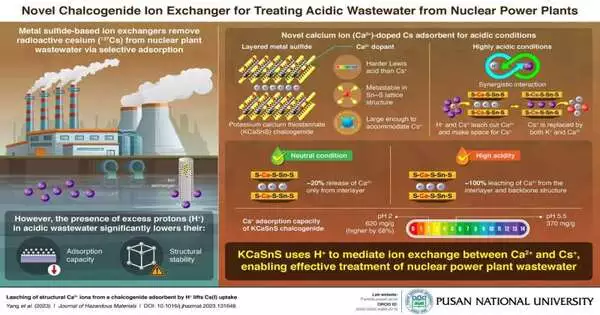
137Cs, an isotope of cesium, is one of the main byproducts of the nuclear fission process used to generate electricity. This radioactive element has a half-life of 30 years and is typically removed from wastewater from nuclear power plants (NPPs) through selective adsorption with ion exchangers. However, acidic wastewater severely hinders this process because excessive protons (H+) damage the adsorbent’s lattice structure and reduce its ability to adsorb.
A group of researchers from Pusan National University in Korea, led by Prof. Kuk Cho, recently discovered a means to turn this obstacle into a benefit. A novel layered calcium (Ca2+)-doped chalcogenide ion exchanger, potassium calcium thiostannate (KCaSnS), was presented in their groundbreaking work in the Journal of Hazardous Materials.
“KCaSnS’s impressive adsorption capacity can help alleviate the challenges associated with managing radioactive waste by providing a practical solution for reducing the volume of radioactive waste produced during spent fuel reprocessing and nuclear power plant decommissioning,”
Prof. Kuk Cho from Pusan National University, Korea
It uses the ordinarily hazardous H+ particles in acidic wastewater to upgrade the cesium particle (Cs+) adsorption process. H+ and Cs+ basically remove the Ca2+ ions from KCaSnS to make room for Cs+.
“By incorporating Ca2+ into the Sn-S matrix, the troublesome proton was transformed through a transformative approach into a functional agent, resulting in a metastable structure. Also, Ca2+ is a harder Lewis acid than Cs+, so it can easily leave the lattice in acidic conditions because it has a weaker affinity for the Lewis soft base. S2 In the study, the team used the hydrothermal process to synthesize the novel KCaSnS ion-exchange material, which was then used to investigate the adsorption of a non-radioactive isotope of Cs+ (to avoid radioactivity exposure) in various solutions with pH values ranging from 1 to 13. “This provides a large enough space for Cs+ to reside after its release from the lattice structure,” Prof. Cho says, referring to the mechanism.
The team discovered that the Cs+ adsorption capacity was 370 mg/g at pH 5.5 (neutral), while it increased to 620 mg/g at pH 2, which is strongly acidic, by 68%. Surprisingly, this trend was very different from what previous studies had shown.
The analysts credited this perception to the way that under nonpartisan circumstances, the Ca2+ was filtered out just from the interlayers, which represented around 20% of the complete spots accessible for Cs+ to be adsorbed by the S2-particles in the Sn-S framework.
On the other hand, more Cs+ ions could enter the lattice due to the leaching of nearly all Ca2+ ions from the interlayer and backbone structures under extremely acidic conditions. In addition, the ion exchange involved interlayer K+ in all instances.
These outcomes lay out KCaSnS as a promising contender for the expulsion of radioactive particles from NPP wastewater. High-performance adsorbents for highly acidic environments may be developed in new ways thanks to the insights gained from this study. “Professor Cho asserts that the impressive adsorption capacity of KCaSnS can provide a practical solution for reducing the volume of radioactive waste produced during spent fuel reprocessing and decommissioning of nuclear power plants, which can help alleviate the challenges associated with managing radioactive waste.”
More information: Chenyang Yang et al, Leaching of structural Ca2+ ions from a chalcogenide adsorbent by H+ lifts Cs(I) uptake, Journal of Hazardous Materials (2023). DOI: 10.1016/j.jhazmat.2023.131648