To fulfill the developing requests of the gadget business, architects and material researchers are attempting to work on the exhibition of battery innovations constantly. Lithium (Li) metal batteries, with a metallic Li anode, are among the most notable and broadly researched battery arrangements.
Nanoengineering techniques and alternative solid or liquid electrolytes have recently been used by researchers to try to improve the performance of these batteries, which have been around for decades. The plan for new encouraging electrolytes could serve to fundamentally improve the security and expand the lifecycle of Li-metal battery cells.
Specialists at Stanford College have as of late acquainted themselves with another technique: high-entropy electrolytes for Li-metal batteries. This methodology, framed in a paper in Nature Energy, permitted them to distinguish promising electrolytes that fundamentally further developed the cycling dependability of batteries at high current densities.
“Recent advances in electrolytes have significantly increased cyclability by improving electrochemical stability at the electrode interfaces, but simultaneously achieving high ionic conductivity has remained challenging. We report an electrolyte design strategy for enhanced lithium metal batteries by increasing the molecular diversity in electrolytes, which essentially results in high-entropy electrolytes.”
Sang Cheol Kim, Jingyang Wang and their colleagues wrote in their paper.
“Ongoing advances in electrolytes have extraordinarily further developed cyclability by improving electrochemical soundness at the anode interfaces; however, simultaneously accomplishing high ionic conductivity has remained challenging,” Sang Cheol Kim, Jingyang Wang, and their partners wrote in their paper. “We report an electrolyte plan methodology for improved lithium metal batteries by expanding the sub-atomic variety in electrolytes, which basically prompts high-entropy electrolytes.”
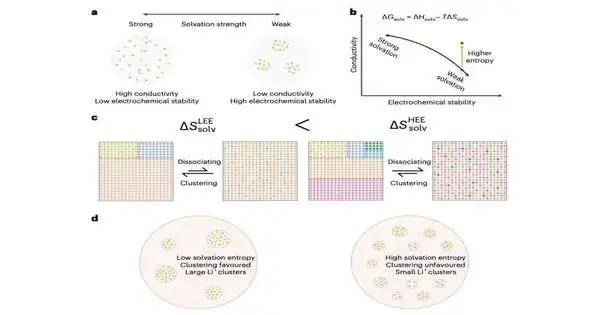
The design of electrolytes with improved ion transport capabilities that do not compromise the stability of Li-metal batteries was the primary goal of the recent work by Kim, Wang, and their colleagues. They came up with a method that can be used to change the structures of solvents in electrolytes that are weakly solvating and have extensive ion clustering in them in order to accomplish this.
As a component of their review, they applied their proposed methodology, utilizing X-beam dispersing strategies and running sub-atomic elements (MD) reenactments (i.e., PC-based recreations of atomic frameworks). This permitted them to recognize qualities that make electrolytes for Li-metal batteries especially ideal, both with regards to strength and ionic conductivity.
“That’s what we find: in pitifully solvating electrolytes, the entropy impact decreases particle grouping while at the same time saving the trademark anion-rich solvation structures, which are portrayed by synchrotron-based X-beam dispersing and sub-atomic element reproductions,” Kim, Wang, and their analysts wrote in their paper.
“Electrolytes with more modestly measured bunches display a twofold improvement in ionic conductivity compared to regular pitifully solvating electrolytes, empowering stable cycling at high current densities up to 2C (6.2 Mama cm2) in without anode LiNi0.6Mn0.2Co0.2 (NMC622)|Cu pocket cells. The adequacy of the plan technique is checked by execution upgrades in three different pitifully solvating electrolyte frameworks.”
The new strategy that Kim, Wang, and their coworkers came up with could soon be used to design a wide variety of promising electrolytes for Li-metal batteries, which would help the ongoing effort to make them perform better. A wide range of electronics, including electric or hybrid vehicles, medical equipment, and other advanced technologies, are powered by these more efficient batteries.
“We imagine that this work can prod endeavors to foster HEEs that can push lithium metal batteries nearer to pragmatic applications and to extensively foster high-entropy arrangements with unrivaled properties for different applications,” the specialists wrote.
More information: Sang Cheol Kim et al, High-entropy electrolytes for practical lithium metal batteries, Nature Energy (2023). DOI: 10.1038/s41560-023-01280-1