The blood-brain barrier (BBB) is a dense, nearly impermeable layer of cells that protects the brain and vital organs from threats to the bloodstream, such as toxins and bacteria, and allows very limited amounts of small molecules to pass through., passing nutrients, for example. However, this shield makes it difficult for researchers to study the brain and develop drugs to treat brain disorders. Now, new research from Caltech has revealed a previously unknown mechanism by which certain viral vectors (protein envelopes designed to carry a variety of essential cargo) can cross the BBB. This mechanistic insight could provide a novel approach to viral vector design for research and therapeutic applications. Understanding these and other emerging mechanisms will allow researchers to understand how new pathogens can exploit the brain’s defenses and develop ways to block them.
The research was conducted in the laboratory of Viviana Grădinaru, professor of neurobiology and bioengineering and director of the Lois and Victor Troendl Center for Molecular and Cellular Neurobiology, part of Caltech’s Tianqiao and Chrissy Chen Neuroscience Institute. Science Advances, 19 April The first author of the study is Timothy Shay, director of research at Caltech’s Beckman Institute’s CLOVER Center. biotechnologist Xiaozhe Ding; and Erin Sullivan, CLOVER Research Associate.
Although the BBB serves as a strong defense for the brain, some viruses have naturally evolved the ability to bypass the BBB. For decades, researchers have studied how to use these viruses as BBB-separating Trojans. To do this, researchers remove the initial viral load of the virus and then use the empty envelope to carry useful therapeutic or research tools.
“To say that an enzyme that regulates blood pH and allows us to taste the fizz in soda is an unlikely target for viruses to cross the BBB is an understatement.” We can now use CA-IV and other fascinating targets that have emerged as a result of our strategy based on uncovering the mechanics of BBB-crossing viral vectors to help us create next-generation viral and non-viral delivery vectors for the brain. And perhaps it will also help us create resilience against emerging infections that may use the same routes to enter the brain.”
Viviana Gradinaru, the Lois and Victor Troendle Professor of Neuroscience and Biological Engineering
Viral vectors capable of crossing the BBB can deliver desired genes to the brain with a simple injection into the bloodstream, eliminating the need for invasive intracerebral administration. Unfortunately, most vectors derived from naturally occurring viruses are very inefficient at crossing the BBB and require high doses, increasing the risk of side effects.
Inspired by nature, Grădinaru’s lab has spent the last decade using directed evolution, a technique developed at Caltech by Nobel laureate Francis Arnold, to direct the evolution of vectors and improve their ability to cross the BBB. Over the years, this group has developed dozens of vectors with different functions that cross the BBB and target different tissues and cell types from different species. Along the way, they noticed that different vectors could behave differently between model organisms, suggesting that each vector identified a different efficient pathway from the bloodstream to the brain.
The researchers knew that these vectors could intersect, but it was still unclear how they intersected. Where is the access point for the BBB citadel wall? In this new study, a team led by Shay, Sullivan, and Ding sought to elucidate these mechanisms using a multidisciplinary approach, each combining the researchers’ expertise in protein chemistry, molecular biology, and data science methods.
First, Shay and Sullivan developed a cell culture screen to rapidly test the ability of dozens of different proteins found on the surface of the BBB to enhance vector infectivity in vessels. Ding then used sophisticated computer models (based on a sophisticated artificial intelligence program called AlphaFold) to simulate how the vectors interact with other proteins, revealing the geometry of the interactions displayed on the screen. They then identified which vectors interacted best with which proteins in a sort of “March Madness” competition (discussed in the next article) and replicated the results of the screen experiments.
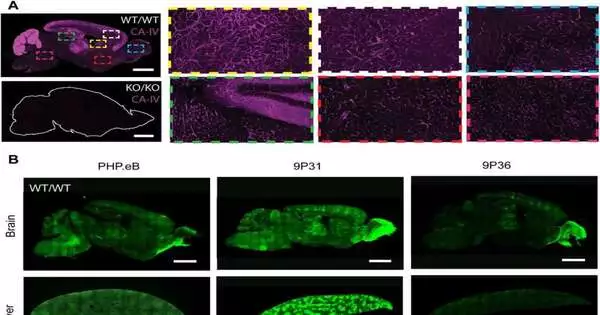
The research team discovered a specific enzyme called carbonic anhydrase IV (CA-IV) that allows several different viral vectors to cross the BBB. Interestingly, CA-IV is an ancient enzyme found in the BBB of various species, including humans. Previously, it was not known to facilitate any type of BBB crossover process. In the future, this combined experimental and computational approach could accelerate the discovery of additional solutions for crossing the BBB, and the team is excited about the potential of using these molecular gates to deliver brain therapy.
“Crossing the gene-brain barrier is an important biological enigma,” says Grădinaru. “It’s an understatement to say that the enzymes that regulate blood pH and give you the taste of gas in soda are counterintuitive targets that help viruses get past BHE. Now we can use CA-IV and other interesting targets. In our view, it relies on elucidating the mechanisms of BBB-crossing viral vectors to aid in generative design. Perhaps this will help develop resistance to new pathogens that can invade the same pathway to the brain.”
Understanding the range of mechanisms by which viral vectors enter the brain is essential to enabling individualized treatment for diverse populations. Their brains and BBB vary greatly from species to species and even from individual to individual. In fact, a person’s BBB can change throughout their life. The discovery of new BBB crossing mechanisms will allow a wide range of neuropharmaceutical delivery options to be tailored to individuals with diverse biological profiles.
More information: Timothy F. Shay et al, Primate-conserved carbonic anhydrase IV and murine-restricted LY6C1 enable blood-brain barrier crossing by engineered viral vectors, Science Advances (2023). DOI: 10.1126/sciadv.adg6618