A review distributed in ACS Applied Materials and Connection Points depicts an original technique for delivering hydrogen peroxide (H2O2) without radiating carbon dioxide (CO2), one of the most ozone-harming substances and one of the world’s most commonly created synthetic compounds.
Hydrogen peroxide is utilized to fade texture, mash, and paper and to brighten teeth. It is likewise utilized as an engine fuel for satellite mind control and as a sanitizer or cleaning agent in medical clinics. Exactly 2 million metric tons of the compound are created every year.
“To comprehend the effect of our discoveries, it’s important most importantly to remember the meaning of H2O2 in the compound business and how it’s right now delivered,” said Ivo Freitas Teixeira, a teacher of science at the Government College of So Carlos (UFSCar) in So Paulo State, Brazil. He has a Ph.D. in inorganic science from the University of So Paulo (USP) and was a Humboldt Individual at the Maximum Planck Establishment of Colloids and Connection Points in Potsdam, Germany, somewhere in the range of 2019 and 2021.
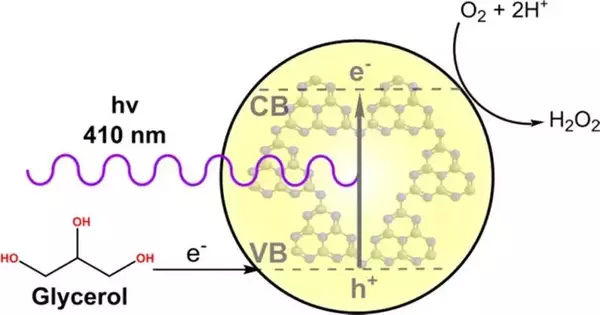
“We reduced O2 via a photocatalytic method in which the hydrogen source was either the water in the reaction media or the sacrificial reagent, which was often glycerol, a byproduct of biodiesel manufacturing,”
Ivo Freitas Teixeira, a professor of chemistry at the Federal University of São Carlos (UFSCar)
“Everything this peroxide is created by is an interaction that includes anthraquinone [a compound got from the hydrolysis of anthracene, a harmful substance].In this cycle, anthraquinone is decreased and then oxidized to make H2O2. The downsides of the strategy are the significant expense of anthraquinone and the utilization of valuable metals, for example, Pd [palladium] and H2 [hydrogen] as decreasing specialists. “This hydrogen is created by steam-methane improvement, which includes high temperatures and delivers CO2, adding to a dangerous atmospheric devastation,” he said.
The experts described how they made peroxide from oxygen (O2) by using photocatalysis to direct the cycle in the review.In photocatalysis, the impetuses (substances that speed up the synthetic response) are actuated by apparent light rather than high temperature or strain. One more benefit of their technique was the utilization of carbon nitride as a photocatalyst.
This material comprises just carbon and nitrogen, the two of which are abundant in Earth’s outermost layer, and can be enacted in the noticeable district, which relates to around 45% of the sun-powered range. It is thus possible that daylight can be used instead of counterfeit lighting, making the cycle more cost-effective.
Subsequent to testing different response conditions, the scientists showed up at a framework with a magnificent pace of H2O2 creation. “We achieved O2 reduction through a photocatalytic course in which the hydrogen source was the water in the response medium or the conciliatory reagent, normally glycerol, a byproduct of biodiesel production,” Teixeira explained.
Carbon nitride is used as a semiconductor in this framework to isolate charges when washed in light, advancing decrease and oxidation responses.The O2 is diminished to H2O2, and the conciliatory reagent (glycerol) is oxidized. The H2O2 is acquired without the need to utilize H2 and, thus, without CO2 discharges.
“The road we needed to travel in our examination until we showed up at the outcomes portrayed in the distributed article was a long one since we found that, at the same time as H2O2 was delivered on the outer layer of the photocatalyst, it could likewise be corrupted,” Teixeira said.
“We needed to play out a few tests and continue to change the photocatalyst to advance the development of H2O2 and keep away from its disintegration.” Understanding the instrument by which H2O2 breaks down on the outer layer of carbon nitride was critical to empowering us to foster the ideal photocatalyst for this response.
More information: Andrea Rogolino et al, Modified Poly(Heptazine Imides): Minimizing H2O2 Decomposition to Maximize Oxygen Reduction, ACS Applied Materials & Interfaces (2022). DOI: 10.1021/acsami.2c14872