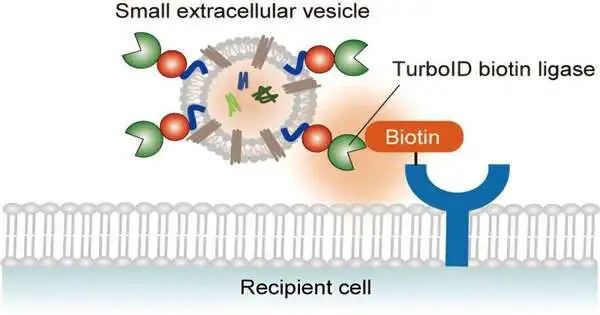
One way that cells speak with each other is through the emission and take-up of extracellular vesicles (EVs). EVs convey a huge number of cargoes, including proteins, lipids, and nucleic acids. Their take-up influences the capability of beneficiary cells by affecting flagging cycles and quality articulation.
Notwithstanding, in spite of a broad investigation of EVs, little is known about their explicit take-up by beneficiary cells.
“Understanding how beneficiary cells take up EVs is basic for unraveling the more extensive components overseeing cell-to-cell correspondence in cell processes in both wellbeing and illness,” makes sense of Koshi Imami of the RIKEN Community for Integrative Clinical Sciences.
Imami and partners have now fostered a clever technique to follow the cooperation among EVs and beneficiary cells. The TurboID-EV framework works by marking beneficiary cell proteins near EVs with biotin (vitamin B7). The examination is distributed in the journal Scientific Science.
“In contrast to conventional methods that employ microscopy or fluorescent protein tagging to target EVs, our approach offers a comprehensive perspective of the proteins involved in EV absorption and interactions within recipient cells,”
Koshi Imami of the RIKEN Center for Integrative Medical Sciences.
“Dissimilar to customary procedures that label EVs with fluorescent proteins or use microscopy, our strategy gives a worldwide perspective on proteins associated with EV take-up and connections inside beneficiary cells,” says Imami.
By distinguishing the biotin-labeled proteins utilizing biochemical enhancement and mass spectrometry, specialists can gather signs of the sub-atomic system’s basic EV take-up.
Imami and partners communicated a biotin ligase that is designed to meld with EV films in human undeveloped kidney cells without disrupting EV emissions. By gathering discharged TurboID-EVs and hatching them with beneficiary cells marked with weighty amino acids and enhanced with biotin, they could look at biotinylation occasions that happen during the take-up of EVs.
The scientists recognized more than 450 biotinylated beneficiary proteins. They included notable ones associated with the cycle by which cells immerse outside substances to get them. The group likewise found proteins engaged with intracellular vehicle and film-related proteins, which could be key for EV take-up in this model.
The technique can be adjusted for various EV subtypes and cell types. “The flexibility of our framework permits specialists to explore the particularity of EV take-up systems in numerous natural settings,” Imami says.
Finding the proteins associated with EV take-up could facilitate how we might interpret how malignant growth cells spread and assist with creating EV-based drug-conveyance frameworks that target explicit cell types.
Imami’s group is presently attempting to apply the TurboID-EV framework to a mouse model to comprehend how disease spreads between organs. “Growth-determined EVs are known to be taken up by organ-explicit cells to get ready for disease to spread to new organs,” Imami makes sense of. “We need to describe the capability of these EVs.”
More information: Yuka Li et al. TurboID-EV: Proteomic Mapping of Recipient Cellular Proteins Proximal to Small Extracellular Vesicles, Analytical Chemistry (2023). DOI: 10.1021/acs.analchem.3c01015