Microscopes are an important tool in biomedical research because they enable for thorough observation and imaging of tissues. Because biological materials are opaque by nature, substantial light scattering occurs as light passes through tissues, resulting in a high amount of background noise and complex optical distortion. As a result, standard light microscopes only allow us to see the surface of the tissues, and features that are numerous cell layers deep are out of reach for many microscopes. This makes getting high-resolution optical pictures of microstructures deep into tissues extremely difficult.
The scanning electron microscope (SEM) provides a high-resolution view of the surface of three-dimensional objects. It operates by scanning an object’s surface with a concentrated beam of electrons and detecting electrons reflected from and knocked off the sample surface. Many objects in transmission microscopy are three-dimensional, meaning they are thicker than the imaging system’s depth of focus. The three-dimensional (3-D) image-intensity distribution is made up of a sequence of two-dimensional images (optical slices) that each focus on a distinct region of the object.
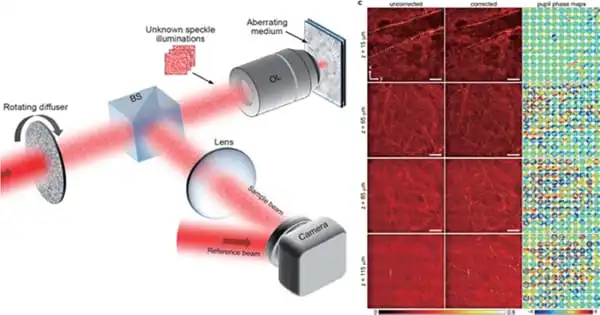
A research group led by Prof. CHOI Wonshik from the Institute of Basic Science’s Center for Molecular Spectroscopy and Dynamics (CMSD) showcased an imaging technique called “reflection matrix microscopy” about a year ago, which combined the powers of both hardware and computational advanced adaptive optics. In contrast to traditional imaging, it measures a reflection matrix that comprises all accessible information about the connection between an imaging system’s input and output fields, including objects of interest. Post-digital image processing can then be used to obtain a clean, undistorted image of the item from the measured matrix.
In the future, quicker reflection matrix imaging technology is predicted to provide real-time, nondestructive 3D optical diagnosis, leading to speedier diagnosis and improvements in brain research. We will continue to improve it so that it may be used in all wave engineering areas, including biomedical imaging.
Prof. CHOI Wonshik
As a result, the technology emerged as a viable option for label-free non-invasive high-resolution optical imaging deep within biological tissues. Most conventional AOs are clearly outperformed by matrix imaging. The researchers, for example, demonstrated that this technique was powerful enough to ‘see through’ an undamaged mouse skull and enable for reliable imaging of neurons beneath.
Despite its outstanding performance, reflection matrix microscopy was not without flaws. Measuring the whole reflection matrix takes time and is susceptible to external disturbances since a large number of interferometric pictures for all accessible input illumination fields must be measured. While more sparse sampling can speed up the process, insufficient sampling can result in a restricted ability to rectify distortions. As a result, real-time volumetric imaging of living samples was not achievable, resulting in practical limitations in its applicability to biodynamic studies.
The same IBS group recently announced a new and enhanced version of their prior AO microscope technology in a paper published in the journal Light: Science & Applications. This revolutionary real-time volumetric AO imaging technique provides 3D imaging over a wide depth range in highly aberrated samples while minimizing picture deterioration.
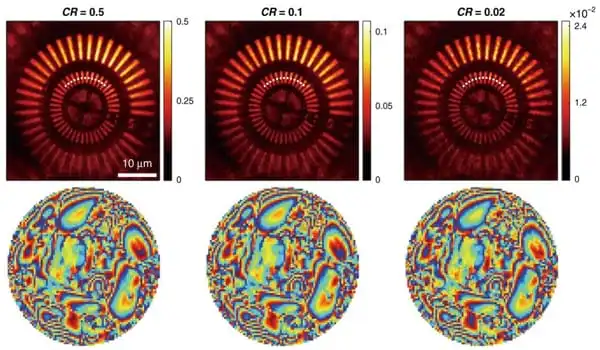
Choi’s team developed a compressed sensing technique in the context of matrix imaging to speed up data collecting. In their previously deployed reflection matrix microscope, they simply added a revolving optical diffuser to progressively illuminate unknown speckle patterns on a material. They then obtained a compressed reflection matrix by acquiring a substantially fewer number of speckle photos than was previously necessary, resulting in a nearly 100-fold reduction in matrix acquisition time.
They used a compressed “time-reversal matrix” and a novel approach in picture post-processing to distinguish sample information and aberrations individually. The advantage of this technology is that it not only drastically reduces matrix acquisition time, but it also eliminates the requirement for precise calibration or specialized illumination pattern selection.
The use of compressed time-reversal matrix imaging enables near-real-time volumetric AO imaging. The capabilities of the new microscope were proven by aberration-free 3D imaging of myelin nerve fibers in a mouse brain. The data acquisition time for volumetric imaging of 128 × 128 × 125 μm3 tissue was only 3.58 seconds, with a diffraction-limited lateral resolution of 0.45 μm and an axial resolution of 2 μm.
It is anticipated that it will pave the way for the practical application of matrix imaging in all domains of wave engineering, including biomedical imaging. Choi, Associate Director, stated, “In the future, quicker reflection matrix imaging technology is predicted to provide real-time, nondestructive 3D optical diagnosis, leading to speedier diagnosis and improvements in brain research. We will continue to improve it so that it may be used in all wave engineering areas, including biomedical imaging.”