Scientists from the University of Groningen have discovered a simple method for delivering previously distant chiral Z-alkenes, particles that provide a critical, manufactured easy route for the development of bioactive atoms.
Rather than eight to ten manufactured moves toward producing these atoms, the new response should be possible in three stages without the requirement for any cleansing. The vitality lies in a phosphine particle that is ordinarily used to make metal-containing impetuses, yet that ends up being the best beginning stage for this synthetic response. The outcomes were distributed in Science Advances on January 13.
Natural mixtures are exceptionally adaptable. Single, double, or triple bonds can connect their carbon molecules.Moreover, numerous naturally significant particles contain chiral focuses, portions of the particle that can be in two identical representation positions, tantamount to a left and a right hand. Particles that have a twofold bond, a chiral focus, and a receptive gathering for engineered changes that are generally close to one another are likewise significant, yet scientists find that these are truly challenging to make.
“The molecule dislikes this and, given the chance, will convert into the more stable E-alkene. This is why it is difficult to synthesize less stable Z-configured alkenes. Z-alkenes are extremely important but also difficult to produce.”
Syuzanna Harutyunyan, Professor of Homogeneous Catalysis at the University of Groningen.
Unstable
Alkenes are compounds that contain two carbon particles linked by a two-way bond. While imagining these two carbon particles on a level plane, we can recognize Z-alkenes, in which both carbon iotas are associated with one more carbon on a similar side (both facing up), and E-alkenes, in which the associated carbons are on inverse sides (one up and one down). Z-alkenes are shaky on the grounds that carbons that are associated on a similar side are compelled to be near one another.
“The particle could do without this, and assuming it gets an opportunity, it will change into the more steady E-alkene.” “To that end, it is difficult to make less steady Z-designed alkenes,” makes sense to Syuzanna Harutyunyan, Teacher of Homogeneous Catalysis at the College of Groningen. “Z-alkenes are exceptionally helpful, yet hard to make.”
The group expected to make the less stable Z-alkenes, where the twofold bond is associated with a chiral carbon focus, and further interface with a profoundly responsive carbon community, which is extremely interesting.
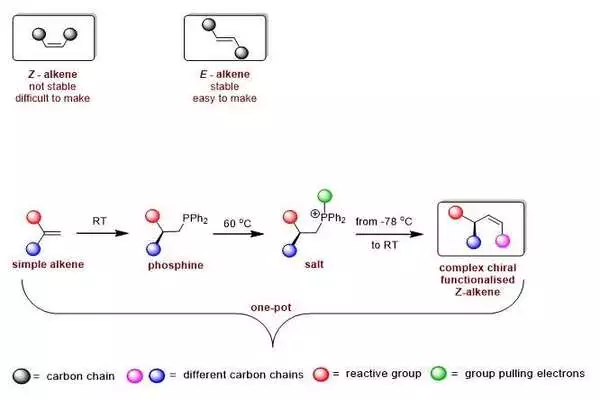
This image shows the setup of Z- and E-alkenes (upper board) and the one-pot combination of functionalized Z-alkenes.
Responsive salt
Utilizing known engineered strategies, it would take around eight to ten separate moves to make such a construction. Harutyunyan and her group attempted to work on this by beginning with a particle called phosphine. According to co-creator Roxana Postolache, “This atom is typically used to deliver metal-containing impetuses.” In past work, we developed a method for making chiral phosphine, which framed the reason for our new engineered course on Z-alkenes.
Harutyunyan states, “We took our phosphine and transformed it into a salt.” This would allow for the formation of a two-way bond with Z-design.
However, this salt is extremely receptive, and all attempts to present a twofold bond resulted in a large number of items that the researchers didn’t require.”In this way, we needed to figure out how to tune the reactivity,” which makes sense of Postolache.
Blackboard
This step necessitated the use of a board and chalk approach, which Harutyunyan and her group employed to evaluate options. A potential arrangement was tracked down by adding a unique gathering to the phosphine to make an alternate sort of salt. According to Harutyunyan, “We calculated that this ought to pull electrons from the phosphorus and would permit us to tune the reactivity.”
First, creator Luo Ge took the thought from the board to the lab. “We attempted to make this thought work, and we got it right with our most memorable endeavor.” “It was an unexpected treat to see that our thinking truly worked.” They thus enhanced the response and afterward utilized their strategy to create genuinely bioactive mixtures.
Possibilities
A major benefit of the new engineered course is that it makes fewer strides and is basically a one-pot response. It simply requires room temperature for the initial step, gentle warming (50–70 °C) to make the salt, and 78 °C for the last step of making the twofold bond with a Z-design.
According to joint first creator Esther Sinnema, “By involving our phosphine as a manufactured device instead of an impetus, we opened up a wide range of conceivable outcomes.” We could make countless new chiral Z-alkenes and utilize the strategy to adjust bioactive mixtures. In the paper, we present 35 unique particles that were made with our strategy.
“We expect that our review will prepare for utilizing financially accessible, straightforward alkenes to make substantially more perplexing functionalized alkenes through phosphine and salt intermediates,” says Harutyunyan.
More information: Luo Ge et al, Enantio- and Z-selective synthesis of functionalized alkenes bearing tertiary allylic stereogenic center, Science Advances (2023). DOI: 10.1126/sciadv.adf8742. www.science.org/doi/10.1126/sciadv.adf8742





