Specialists from Skoltech, Jiangsu Ordinary College, and somewhere else have anticipated surprising mixtures framed by lithium and cesium under high tension. These brand-new substances possess the highly sought-after property of superconductivity, which means that they lose any electrical resistance at critical temperatures between minus 223 and minus 213 degrees Celsius. In addition, they have crystal structures that have never been seen before. The research was published in Nano Letters.
According to conventional chemistry, lithium and cesium do not combine in any way. Yet, it would seem that on the off chance that you put them under tension, a few mixtures do arise. Some of them had been anticipated previously; yet another concentrate by a group of Chinese, Russian, and U.S. researchers utilized a more solid calculation and recognized new and more steady stages.
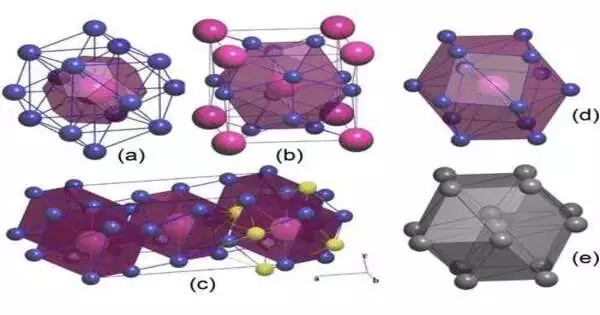
Four lithium-rich compounds with seemingly bizarre formulas, such as Li14Cs, that you won’t find in a chemistry textbook, were discovered by the researchers using fundamental theoretical principles and the USPEX crystal structure prediction algorithm, which was previously developed by study co-author and Skoltech Professor Artem R. Oganov.
“The most electropositive element in the periodic table as most people know it, cesium, is meant to give away electrons under normal circumstances, thus if anything, we would anticipate lithium to attract cesium’s electrons.”
Skoltech Professor Artem R. Oganov
“We would expect lithium to attract the electrons of cesium, which is the most electropositive element in the periodic table as most people know it, under normal circumstances. It is supposed to give up electrons,” Oganov said.
A chemical element’s atom’s electro positivity is a fundamental property that describes how eagerly it sheds electrons or, in the case of electronegativity, retains them. Oganov and his colleagues disrupted the periodic table by expanding the concept of electronegativity to high pressures.
The researcher continued, “Yet under pressure, it is the other way around.” Four new compounds are formed as a result of cesium’s unusual chemical behavior in grabbing lithium’s electrons. Two of these compounds, Li14Cs and Li6Cs, turn out to have crystal structure topologies previously unknown. This is a genuinely interesting thing for mixtures of only two components.” Oganov continued, “But here we are, with two new topologies in one binary system.”
The four new lithium and cesium compounds, according to the team, should be able to conduct electricity with no resistance. That is, they are known as superconductors below a certain critical temperature, which can be anywhere from minus 223 to minus 213 degrees Celsius, depending on the compound. Scientists are looking for these materials in the hopes that they will one day enable power grids with unprecedented efficiency, ultrafast microchips, and electromagnets that are strong enough to levitate trains or even control fusion reactors.
“Certainly, from a mechanical viewpoint, these basic temperatures are no better contrasted with what we’ve seen in polyhydrides — the hydrogen-rich mixtures of certain metals.” However, this study deepens our understanding of lithium chemistry, and lithium as such could be interesting for superconductivity, perhaps in the form of a hypothetical ‘lithide’ compound—so far we don’t know if it exists or how to spell it,” Oganov stated, explaining that the lithium atom is very similar to that of hydrogen and could therefore serve as a substitute for hydrogen in a compound that is similar to a polyhydride.
Lithium, like hydrogen, has one valence electron. It is also one of the lightest elements, which makes it good for superconductivity. It is common knowledge that a superconducting atom’s critical temperature rises in tandem with its mass.
The Dong-Oganov reinvented electronegativity scale, which was published last year, predicted the electronegativity inversion reported in this study—namely, lithium giving up electrons to cesium—like many other high-pressure chemistry anomalies.
More information: Hong-Mei Huang et al, Novel Topological Motifs and Superconductivity in Li-Cs System, Nano Letters (2023). DOI: 10.1021/acs.nanolett.3c00875