Cedars-Sinai Malignant growth specialists have utilized an extraordinary accuracy medication and a man-made brainpower (computer-based intelligence) instrument called the Sub-atomic Twin Accuracy Oncology Stage to distinguish biomarkers that beat the standard test for foreseeing pancreatic disease endurance. Their review, distributed in Nature Disease, shows the reasonability of a device that might one day, at some point, guide and further develop therapy for all malignant growth patients.
“Sub-atomic Twin, which we created at Cedars-Sinai, can be utilized to concentrate on any growth type, including pancreatic disease, which is famously hard to treat,” said Dan Theodorescu, MD, Ph.D., head of Cedars-Sinai Malignant Growth and the Stage ONE Establishment Recognized Seat, and senior creator of the review. “Utilizing our Atomic Twin innovation, we expect to make tests that can be involved even in areas that need admittance to cutting-edge assets and innovation, matching patients with the best treatments, and extending the accessibility of accurate medication.”
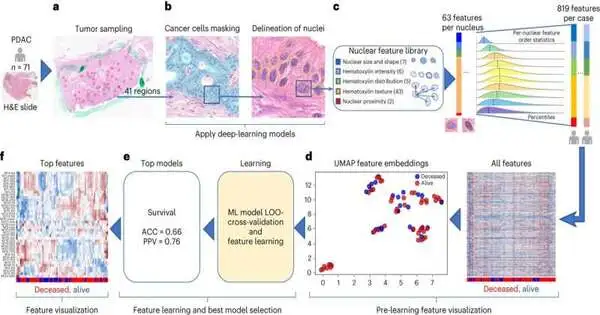
Specialists utilized the sub-atomic twin stage to examine blood and tissue tests from 74 patients with the most well-known and most forceful pancreatic disease type, pancreatic ductal adenocarcinoma. The sickness starts in the cells lining the pipes that convey stomach-related compounds from the pancreas to the small digestive system.
“We had already started a thorough process of gathering tissue and blood samples from pancreatic cancer patients, which provided us with an excellent chance to test the Molecular Twin platform. Molecular Twin will become an even more powerful tool when we add more patients to the platform, not only for pancreatic cancer but for all malignancies.”
Arsen Osipov, MD, assistant professor of Medicine, program lead in the Pancreatic Cancer Multidisciplinary Clinic.
Specialists originally joined 6,363 different organic data sets of interest, including hereditary and sub-atomic data, to make a model that precisely anticipated sickness endurance in 87% of patients. The group then utilized simulated intelligence to smooth out the information and make a model that performed almost as well with only 589 pieces of information. Focusing much further, specialists established that proteins found in the blood were the best single indicator of pancreatic disease endurance.
The full and smoothed-out models and the blood-protein test beat the main Food and Medication Organization-endorsed pancreatic malignant growth test, a blood test called CA 19–9. The discoveries were approved in autonomous datasets from The Malignant Growth Genome Chart book, Massachusetts General Clinic, and Johns Hopkins College.
The Sub-atomic Twin stage was sent off by Cedars-Sinai in 2021, said Arsen Osipov, MD, collaborator teacher of medication, program lead in the Pancreatic Malignant Growth Multidisciplinary Facility and Accuracy Medication Program at Cedars-Sinai Disease, and first creator of the review.
“There’s an enormously neglected need for the improvement of biomarkers to direct our therapy of pancreatic malignant growth,” Osipov said. “We had proactively embraced a complete assortment of blood and tissue tests from patients with pancreatic disease, and this offered us a decent chance to test the sub-atomic twin stage. As we develop the stage with additional patients, Sub-atomic Twin will turn into a significantly more vigorous apparatus in pancreatic disease, yet across all malignant growths.”
Jennifer Van Eyk, Ph.D., a specialist in the investigation of proteins, overseer of the High Level Clinical Biosystems Organization in the Division of Biomedical Sciences at Cedars-Sinai, and a critical individual from the Sub-atomic Twin group, expressed that while hereditary data is useful in foreseeing a patient’s gamble of creating malignant growth and the subtyping of the disease, this study shows that proteins are the way to anticipating patient endurance.
More information: Arsen Osipov et al, The Molecular Twin artificial-intelligence platform integrates multi-omic data to predict outcomes for pancreatic adenocarcinoma patients, Nature Cancer (2024). DOI: 10.1038/s43018-023-00697-7