In a work of systematic biology that advances the discipline, University of Alabama at Birmingham researchers have found 16 unique cell populations in a complex part of the midbrain known as the ventral tegmental area, or VTA.
The VTA plays a key role in dopamine neurotransmission, which is implicated in reward-directed behavior. Substance use disorders are characterized by dysregulation of these reward pathways, which leads to persistent drug-seeking despite negative consequences. In the most recent year, there were over 100,000 drug overdose deaths in the United States. The VTA is also involved in a number of other neuropsychiatric illnesses.
Thus, increasing understanding of its function is a first step in explaining the causes of substance use disorders involving drugs such as cocaine, alcohol, opioids, and nicotine, as well as psychiatric illnesses such as schizophrenia and attention deficit hyperactivity disorder, or ADHD.
Dopamine is a neurotransmitter that the brain uses as a chemical messenger to transfer signals between nerve cells. While decades of research has concentrated on dopaminergic neurotransmission in the VTA, there is also strong evidence for the role of GABA and glutamate, two additional neurotransmitters functioning in the VTA in reward-related behaviors. There is also evidence for “combinatorial” neurons, which have the ability to produce and release several neurotransmitters. These findings point to a new level of complexity in VTA cellular and synaptic function.
“For example, previous single-cell sequencing studies were conducted exclusively in the mouse brain and have relied primarily on sequencing a subset of fluorescence-activated cell sorting-isolated midbrain dopaminergic populations, rather than sampling all VTA cell types, Notably, our sequencing dataset focuses exclusively on VTA sub-regions, unlike other studies that have focused on pooled cells from the mouse substantia nigra and VTA or a subset of fluorescently tagged cells from general midbrain regions.”
Day said
The study of categorization is known as systematic biology, and it usually relates to the classification of species based on their natural relationships. The UAB VTA project classifies cell populations in order to extend and deepen prior work on the many cell types in the VTA and to provide a starting point for decoding the interactions between these cells and their vast connections to other areas of the brain. The study was conducted by co-first authors Robert A. Phillips III and Jennifer J. Tuscher, Ph.D., and corresponding author Jeremy J. Day, Ph.D., and was published in Cell Reports.
After single-nucleus RNA sequencing of 21,600 cells from the rat VTA, the 16 unique cell types were identified by changes in gene expression, resulting in a searchable online atlas of the VTA. The rat is the most commonly used model in reward and substance use research. In contrast to earlier research that used subsets of cells for RNA sequencing, this unbiased approach was used to construct the largest and most thorough single-cell transcriptomic analysis focused solely on the composition and molecular architecture of the VTA.
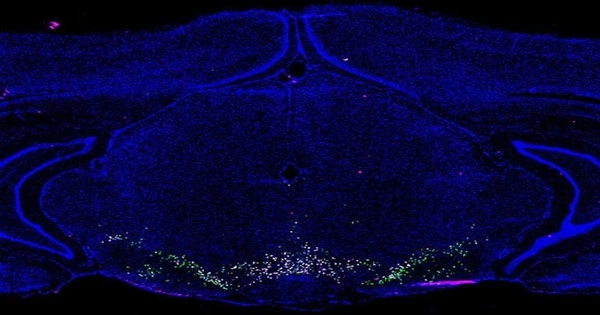
Though it was previously known that the VTA is made up of a variety of cell types, the UAB atlas extends prior studies in several significant ways.
For example, earlier single-cell sequencing investigations have been undertaken only in the mouse brain and have predominantly relied on sequencing a subset of fluorescence-activated cell sorting-isolated midbrain dopaminergic populations rather than sampling all VTA cell types, Day explained. Notably, unlike previous research that has focused on pooled cells from the mouse substantia nigra and VTA or a subset of fluorescently tagged cells from broad midbrain regions, our sequencing dataset focuses entirely on VTA sub-regions.
Classic dopaminergic neurons, three subsets of glutamatergic neurons, three subsets of GABAergic neurons, and nine other cell types, including astrocytes and glial cells, are among the 16 unique cell groups.
The UAB researchers detected four sub-clusters after sub-clusing neuronal cells that may indicate neurons capable of combinatorial neurotransmitter release. They also discovered selected gene markers for both classically defined dopamine neurons and combinatorial neurons. For functional research, a selective marker enables viral targeting of various VTA subclasses.
The researchers also looked at sub-clusters for opioid neuropeptides and receptors and discovered pan-neuronal enhanced expression for risk genes linked to schizophrenia and “smoking initiation,” as well as enrichment of ADHD risk genes in two glutamatergic neuronal populations.
In addition to Day, Phillips, and Tuscher, Samantha L. Black, Emma Andraka, and N. Dalton Fitzgerald from the UAB Department of Neurobiology and Evelyn F. McKnight Brain Institute, and Lara Ianov from the Civitan International Research Center at UAB, are co-authors on the study, “An atlas of transcriptionally defined cell populations in the rat ventral tegmental area.” Day, Phillips, and Tuscher are associate professors, graduate students, and postdoctoral fellows at the UAB Department of Neurobiology, respectively.